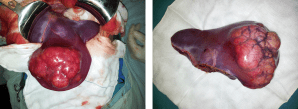
Anatomia Cirúrgica Pancreática
A anatomia cirúrgica do pâncreas é um dos tópicos mais fascinantes e desafiadores da cirurgia do aparelho digestivo. Conhecer detalhadamente a estrutura e a topografia do pâncreas é crucial para a realização de procedimentos cirúrgicos seguros e eficazes. Neste artigo, vamos explorar os aspectos mais importantes da anatomia pancreática, abordando pontos-chave para estudantes de medicina e residentes de cirurgia do aparelho digestivo.
Estrutura Anatômica do Pâncreas
O pâncreas é uma glândula retroperitoneal situada transversalmente no abdome, na altura das vértebras L1-L2, abrangendo os principais vasos sanguíneos e a coluna vertebral. Ele é composto por cinco partes principais: cabeça, colo, corpo, cauda e processo uncinado.
- Cabeça: Localizada à direita da linha média, cercada pelo duodeno. A cabeça do pâncreas possui uma extensão inferior chamada processo uncinado, que está intimamente relacionada com a veia mesentérica superior e a artéria mesentérica superior.
- Colo: Uma parte estreita entre a cabeça e o corpo, situada anteriormente à veia porta, definida pela localização anatômica anterior à formação da veia porta, geralmente pela confluência das veias mesentérica superior e esplênica.
- Corpo: Estende-se para a esquerda, posterior ao estômago, com a superfície anterior coberta pelo peritônio, formando parte da parede posterior do saco menor.
- Cauda: Parte terminal que se estende até o hilo esplênico, confinada entre as camadas do ligamento esplenorrenal juntamente com a artéria esplênica e a origem da veia esplênica.
- Processo Uncinado: Considerado uma parte distinta do pâncreas devido à sua origem embriológica diferente, estendendo-se posteriormente aos vasos mesentéricos superiores.
Peso e Dimensões Normais
Em um adulto saudável, o pâncreas tem um peso médio de aproximadamente 80 a 120 gramas. As dimensões normais do pâncreas variam, mas geralmente medem cerca de 15 a 20 cm de comprimento, 4 a 5 cm de largura na cabeça, e 1,5 a 2,5 cm de espessura.
Vascularização do Pâncreas
A vascularização do pâncreas é complexa e vital para a compreensão cirúrgica. As principais artérias que irrigam o pâncreas são:
- Artéria Pancreatoduodenal Superior: Ramo da artéria gastroduodenal que irriga a cabeça do pâncreas.
- Artéria Pancreatoduodenal Inferior: Ramo da artéria mesentérica superior que também fornece sangue à cabeça do pâncreas.
- Ramos Pancreáticos da Artéria Esplênica: Irrigam o corpo e a cauda do pâncreas.
As veias pancreáticas drenam para a veia esplênica, veia mesentérica superior e, eventualmente, para a veia porta.
Ductos Pancreáticos
O pâncreas possui dois principais ductos: o ducto pancreático principal (ducto de Wirsung) e o ducto pancreático acessório (ducto de Santorini).
- Ducto Pancreático Principal (Ducto de Wirsung): Começa na cauda do pâncreas e percorre o corpo até a cabeça, onde geralmente se junta ao ducto biliar comum na ampola de Vater, regulada pelo esfíncter de Oddi. O diâmetro normal do ducto pancreático principal varia de 1 mm na cauda até 3 mm na cabeça.
- Ducto Pancreático Acessório (Ducto de Santorini): Corre superior e paralelo ao ducto de Wirsung, drenando parte da cabeça do pâncreas na papila duodenal menor.
O esfíncter de Oddi é uma estrutura única de fibras musculares lisas que regula o fluxo das secreções biliares e pancreáticas para o duodeno e impede o refluxo do conteúdo intestinal para o sistema ductal pancreatobiliar.
Considerações Cirúrgicas
Entender a anatomia pancreática é essencial para evitar complicações durante procedimentos cirúrgicos. Algumas das considerações importantes incluem:
- Ressecção Pancreática: Procedimentos como a pancreatoduodenectomia (procedimento de Whipple) requerem um conhecimento detalhado das relações anatômicas para garantir a remoção eficaz do tumor com a menor morbidade possível.
- Drenagem Biliar: A proximidade do pâncreas com o ducto biliar comum exige precisão ao evitar danos durante as cirurgias.
- Anastomoses Pancreáticas: A criação de anastomoses seguras entre o pâncreas e o intestino é crítica para prevenir complicações pós-operatórias, como fístulas pancreáticas.
Variações Anatômicas
As variações anatômicas do pâncreas e dos ductos pancreáticos são comuns e podem impactar significativamente a abordagem cirúrgica. Uma compreensão detalhada dessas variações é essencial para a personalização do plano cirúrgico. Por exemplo, o pâncreas anular é uma condição em que o tecido pancreático forma um anel ao redor do duodeno, podendo causar estenose duodenal.
Conclusão
A anatomia cirúrgica pancreática é um campo complexo e detalhado que exige estudo e prática contínuos. Compreender as nuances dessa anatomia é fundamental para qualquer cirurgião do aparelho digestivo. Como disse o renomado anatomista Andreas Vesalius: “A anatomia é a fundação de todas as ciências médicas.”
Gostou? Nos deixe um comentário ✍️, ou mande sua dúvida pelo 💬 Chat On-line em nossa DM do Instagram.
Hashtags
#AnatomiaPancreática #CirurgiaDigestiva #EstudantesDeMedicina #ResidentesDeCirurgia #Pancreas

Nutritional Management of Acute Pancreatitis
Acute pancreatitis is a common intra-abdominal inflammatory condition of varied aetiology. The disease is mild in the vast majority of patients and has a favourable outcome. The acute severe form of the disease on the other hand is a lethal form with a high mortality and morbidity. A number of strategies have provided clinical benefit in severe acute pancreatitis (SAP). Of these, nutritional management is by far the most effective. SAP is associated with persistent end-organ failure, commonly respiratory, circulatory and renal. Treatment is targeted to support these organs. As of now there is no definitive therapy for acute pancreatitis. Patients are managed with fluids, analgesics, antibiotics and nutritional supplements besides adequately treating local complications such as pseudocyst and walled-off pancreatic necrosis by suitable interventional methods, be it endoscopic or percutaneous. The focus here is nutritional support in the management of SAP.
Which Form of Nutrition: Parenteral or Enteral?
This depends largely on the functional integrity of the stomach and small intestine. Patients of SAP often have poor gastric emptying and paralytic ileus, which is made worse with the use of narcotics. Moreover, local complications of pancreatitis (peripancreatic fluid collections) can have a pressure effect on the stomach and/or duodenum. As a result oral feeds may not be possible in these patients. Patients on ventilator support also cannot be given oral feeds.
Enteral feeding through the nasogastric or nasojejunal tubes is often not tolerated by patients because of discomfort. In addition, these tubes often get displaced or withdrawn. Reinsertion of the tubes, under endoscopic or radiological guidance, is cumbersome in such patients. All these factors favour parenteral feeding. The distinct advantage of enteral nutrition is that it prevents mucosal atrophy and transmigration of bacteria (an important causeof sepsis in SAP). Also, enteral feeding augments intestinal motility and is cheaper than parenteral preparations. Enteral nutrition improves motility in patients with paralytic ileus. The relative merits of these forms of nutritional therapy have been evaluated in a systematic review. Eight published randomized trials including a total of 348 patients were included. Enteral feeding was given through a nasojejunal tube and parenteral nutrition through a catheter placed in a central vein. Enteral nutrition was shown to reduce mortality, multi-organ failure, systemic infection and surgical intervention in comparison with parenteral nutrition. The length of hospital stay too was shown to be reduced. In view of these, enteral nutrition appears to be a better option while managing patients of SAP and has been recommended by the American College of Gastroenterology, American Gastroenterological Association and International Association of Pancreatology.
When should enteral feeding be started?
Patients with mild acute pancreatitis can usually be started on oral feeds in 2–3 days. Those with moderately severe acute pancreatitis can be started on oral feeding only after a variable period and hence should receive enteral nutritional support. Early enteral feeding has been shown to avoid end-organ failure in a large series of patients (1200).
Enteral feeding started within 48 h of onset of illness was associated with organ failure in 21% of patients as opposed to 81% when enteral feeding was started after 48 h. This benefit of early enteral feeding has also been shown in a recent meta-analysis. However, there was no benefit in mortality with early enteral feeding. In yet another randomized controlled trial, early enteral feeding (within 24 h) was compared with on-demand enteral feeding after 72 h.
The primary endpoint of this study was major infection or death. The study did not detect any significant difference in the primary endpoint in either group (early or on-demand feeding). However, it did show that patients receiving on-demand nutrition tolerated oral feeds without using a tube.
- Nasogastric or Nasojejunal
Should the feed be administered in the stomach through a nasogastric (NG) tube or in the jejunum through a nasojejunal (NJ) tube? Gastric feeding is thought to increase pain and aggravate pancreatitis due to food-induced pancreatic stimulation. In view of this, NJ feeding is practised. However, placement of a NJ tube is cumbersome and needs a skilled endoscopist or radiologist. It causes more inconvenience to patients. A nasogastric (NG) tube is thus an alternative. A number of studies have been published comparing NG and NJ feeding. The results of these studies can be summarized as follows: There was no difference in mortality. Feeds were equally tolerated in the two groups and NG feeding is simple. NG feed was not shown to increase pain and is thus as good as NJ feeding. A meta-analysis subsequently published showed no difference in mortality, hospital stay and infection rate between the two groups. Both forms of feeding were equally well tolerated. NJ feeding thus is not advised in the management of most patients with SAP. However, it still has a place when the patient has a high risk of aspiration. Also, patients on a ventilator and those not tolerating NG feed should be fed through NJ tube. The other issue concerning enteral feeding in SAP is the composition of the feed.
- Type of Formulation
Various commercially available formulations include (1) polymeric formulations comprising complex lipids, carbohydrates and proteins and (2) elemental formulations comprising simple amino acids, carbohydrates and free fatty acids. Other formulations used are glutamine-rich feeds and feeds with probiotics, fibres, etc. Immuno-nutrition using arginine, glutamine and polyunsaturated fatty acids has been evaluated in multiple studies and compared with standard feeding. A metaanalysis showed some benefit in mortality but not for prevention of infection, end-organ failure or inflammatory response. This benefit was not seen with the use of probiotics or fibre-based feeds. A systematic review did not show any benefit of immuno-nutrition or probiotics. It also showed that polymeric formulations are as well tolerated as oligomeric ones (elemental).
ERCP Induced Perforations
In the epoch of minimally invasive management of biliary and pancreatic disorders, endoscopic retrograde cholangiopancreatography (ERCP) combined with endoscopic sphincterotomy (ES) has become a prevalent procedure all over the world. Even though ES is a safe procedure, it carries a small but significant number of serious complications which include pancreatitis, bleeding, cholangitis and perforation. As per old literature, ERCP-related perforations were reported in 0.5–2.1% of sphincterotomies with a mortality rate of 16–18%. However, the improvement in the experience and skill of the endoscopy specialists combined with advancements in technology have reduced the incidence of perforation to <0.5% over the years. Sphincterotomy (56%) and guidewire manipulation (23%) are widespread causes of perforations related to endoscopic retrograde cholangiopancreatography (ERCP). There is a dearth of evidence-based strategies with respect to the proper management of ERCP perforations. While one set of investigators promote on-demand conservative and surgical management, based on a clinical course, the others support operative repair in all cases on account of the complications associated with the delayed operative intervention.
INDICATIONS OF SURGICAL MANAGEMENT
1. Large extravasation of contrast at the time of ERCP defined as incomplete dissipation of contrast after 1 min on follow-up plain film.
2. If there is only a small amount of contrast extravasation, where there is complete dissipation after 1 min of ERCP, on follow-up plain film, then a UGI with contrast injection on fluoroscopy is performed in 2–8 h. If this shows extravasation, we recommend surgical exploration.
3. Follow-up CT scan showing a collection due to perforation in the retroperitoneum or intraperitoneum.
4. Retained hardware unable to be removed by endoscopy along with perforation.
5. Massive subcutaneous emphysema.
6. Failure of conservative management.
A delay in diagnosis or in surgery will lead to death. The reason is that there is a massive autodigestion of body tissues which is due to a constant release of enzymes, and this eventually leads to sepsis. The principle of treatment by surgery is the same as endoscopic treatment. Any case that is suspected to have ERCP-induced perforation is kept nil by mouth, and the gastric contents are decompressed by Ryles tube and intravenous antibiotics.
This is done by diverting bile, enteric and pancreatic juices away from the site of perforation. However simple drainage will also cause the juices to flow through the perforation site and body cavities before draining out of the tubes. This could be avoided by diverting the juices through well-controlled different paths which could be done by the following procedures:
1. T-tube in CBD;
2. Placement of duodenostomy tube—lateral/end duodenostomy;
3. Duodenal diverticulization;
4. Pyloric exclusion;
5. Roux-en-Y duodenojejunostomy.
The disadvantage of using Roux-en-Y duodenojejunostomy is that if the edges are inflamed, then the sutures will not hold properly. However other procedures can be used even when the edges are inflamed. Even though duodenostomy appears to be simple, a part of gastric and duodenal contents pass across the perforation site.
Duodenal diverticulization involves three things: (1) tube to divert duodenal and pancreatic juice, (2) T-tube in CBD to divert bile and (3) distal
gastrectomy and Billroth II anastomosis to provide an alternate pathway for food and gastric juice, thereby preventing these from passing through the site of perforation. Although this procedure has been proved to be successful, it is less widely used due to its complex nature. Pyloric exclusion is a simpler form in which the pylorus is closed by purse string by long-standing absorbing sutures like PDS 2.0 instead of distal gastrectomy. Similar to duodenal diverticulization, T-tube drainage of the CBD and loop gastrojejunostomy are done. The duodenal perforation is closed over a duodenostomy tube.
Whenever there is collection which is localized to the retroperitoneum, retroperitoneal surgical approach can be carried out. Advantages of this procedure are (1) it permits gravitational drainage, (2) avoids septic complication of the peritoneal cavity, (3) directs retroperitoneal necrosectomy with post-operative washes and (4) avoids complex intra-abdominal surgeries. However the disadvantage of this procedure is that it can be used only for retroperitoneal-contained perforations.
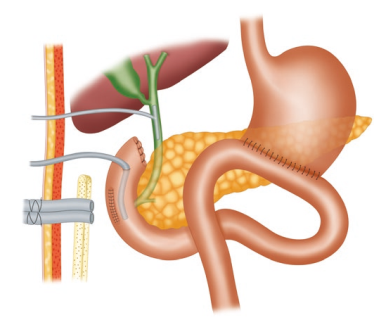
Surgical Management of Retroperitoneal Sarcoma
The most critical component of the treatment of Retroperitineal Sarcoma (RPS) remains the surgical excision, and the best chance for cure is at the time of primary surgery. Surgery should achieve a macroscopically complete excision of the tumor (R0 or R1), minimizing marginality, ideally through an en-bloc resection of all potentially involved structures as determined by careful preoperative imaging in combination with intraoperative findings.
Operative planning also includes the functional assessment of critical organs—eg, the function of each kidney. Contraindications to primary resection are believed to be bilateral renal involvement; encasement of the superior mesenteric artery, celiac axis, and porta hepatis; and spinal cord involvement.
When planning for surgery, it is paramount to take into consideration the histology of the RPS as well as its predicted behavior pattern, as these differ widely. Indeed, the largest transatlantic multi-institutional series identified histologic subtype as a predictor of patterns of local and distant recurrence. Moreover, analysis of a large, single-institution database demonstrated that histologic subtype is the strongest predictor of disease-specific death and affects both local and distant recurrence. Of greater interest, the patterns of contiguous organ involvement are also heavily dependent on histologic subtype.
In light of these data, surgeons oncologists should decide the extent of surgical resection in a multidisciplinary setting at a specialized center after review of imaging and pathology, given that the pattern of growth and prognostic risks vary broadly among the different histologic subtypes. For example, liposarcoma is the histologic subtype with the highest recurrence rate. In addition, it is the one with the least clear separation from normal retroperitoneal fat, given that the well-differentiated component of liposarcoma is virtually undistinguishable from normal fat. As a consequence, the extent of surgery should be aimed at removing all ipsilateral retroperitoneal fat en bloc with the mass at the price of sacrificing at least the ipsilateral kidney and colon and part of or the entire psoas muscle.
A staged approach can be followed in virtually all cases. The stages include:
A. Generous laparotomy, exploration, and retraction.
B. Division of the gastrocolic ligament, division of the transverse colon (plus distal ileum if on the right side), and assessment of the duodenum/head of the pancreas if on the right side, or body/tail of the pancreas and spleen if on the left side.
C. Liberation of duodenum/head of the pancreas if on the right side or body/tail of the pancreas and spleen and duodenojejunal junction if on the left side (when possible) and partial duodenal resection or pancreaticoduodenectomy (< 5% of right-sided retroperitoneal sarcomas) if on the right side or distal pancreatectomy and splenectomy if on the left (40%–50% of left-sided retroperitoneal sarcomas), when too adherent/invaded by the tumor.
D. Dissection of the inferior vena cava (IVC) if on the right side or aorta if on the left side, ligating ipsilateral renal vessels and other collaterals and dissection of the iliac vessels.
E. Peritonectomy, resection of the psoas muscle in the pelvis (plus rectal resection if on the left side) after identification and liberation of the femoral nerve (unless directly invaded) and possibly of the femoral cutaneous branch, while the genitofemoralis and ilioinguinal nerves are usually resected, as these lie between the tumor and the psoas fascia.
F. Section of the origin of the psoas major from the spine, sparing the roots of the femoral nerve and possibly the iliohypogastric nerve, liberation/resection of the costodiaphragmatic fold, and removal of the specimen.
Subcapsular liver dissection or partial hepatectomy are rarely needed for tumors located on the right side, whereas a complete liberation of the right liver lobe is usually of help. Similarly, sleeve gastrectomy or proximal gastrectomy is rarely required for tumors located on the left side. Finally, vascular resections (predominantly iliac vessels on either side and IVC on the right side) are required in 4% of cases.
Leiomyosarcoma and other rarer histologic subtypes such as solitary fibrous tumor are much more well-defined tumors. Their border can be clearly separated from retroperitoneal fat/structures. A wide resection is still required but not necessarily involving the adjacent organs if these are not clearly invaded.
Extended surgery may raise concern for added morbidity. A recent multi-institutional collaboration, however, found that a radical resection is safe and is associated with low 30-day mortality (1.8%). Severe complications were associated with increased age, transfusion requirements, and organ resection score, with a more pronounced risk in patients undergoing splenectomy and pancreatectomy and Whipple procedure.
Although major vascular resection (MVR) is associated with higher morbidity, vascular involvement does not preclude resection because it can be safely performed in specialized centers. MVR may be necessary either due to the origin of the RPS, as is the case for leiomyosarcoma of the IVC, or due to local invasion and involvement. Whereas multiple strategies for approaching MVR can be used, a good understanding of the vasculature and collaterals is critical prior to attempting resection and reconstruction, given that IVC resections are well tolerated if a good network of collaterals is present.
In essence, resection of RPS requires technical expertise in multiple sites throughout the abdominal and pelvic cavity, including the handling of large vessels. Single organ/site expertise is not sufficient. The ability to orchestrate a team of complementary surgical experts is critical to successful management of RPS patients. To minimize the risk of intraoperative and perioperative morbidity, RPS resection should be undertaken by surgical teams with expertise in specific aspects of the anatomy of the retroperitoneal space—for example, expertise in retroperitoneal autonomic and somatic nerves, the lymphatic system, paravertebral vessels, and organs of the gastrointestinal tract.
Required expertise also includes experience with additional procedures, such as full-thickness thoracoabdominal wall resection and reconstruction, diaphragmatic resection and reconstruction, major vascular resection and reconstruction, and bone resection. Surgical teams with these abilities, which may accrue from prior participation in multidisciplinary surgical teams, can achieve macroscopically complete tumor resection in the majority of patients.
Hepatic Hemangioma: Is There an Indication for Surgical Resection?
Hepatic hemangioma (HH) is the most common benign liver tumor. It consists of blood-filled cavities fed by the hepatic arterial circulation, with walls lined by a single layer of endothelial cells, a veritable chaotic entanglement of distorted blood vessels confined to a region as small as a few mm and as large as 10 cm, 20 cm and even 40 cm. The frequency is higher among adults, with a prevalent age at the initial diagnostic in the range of 30-50 years. Literature places the HH incidence at 0.4% to 20% of the total population. At necropsy, the frequency is of 0.4 to 7.3%, all the authors agreeing with an incidence of over 7%. The HH prevalence in the general population varies greatly, most often being discovered incidentally during imaging investigations for various unrelated pathologies. Regarding sex distribution, it seems that women are more susceptible, as confirmed by all pertaining studies, with a reported 4.5:1 to 5:1 ratio of female to male cases. Most often, HH are mono-lesions but multiple-lesions are possible; they account for 2.3% and up to 20-30% of the cases, depending on the source. At the initial diagnosis, the majority of HH measure below 3 cm in size, the so-called capillary hemangiomas; of these, only 10% undergo a size increase with time, for reasons still unknown. The next size class covers lesions between 3 cm and 10 cm in size, referred to as medium hemangiomas. Lastly, giant or cavernous hemangiomas measure up to 10 cm, with occasional literature reports of giant HH reaching 20-40+ cm in size. Location-wise they are most often found in the right liver lobe, often in segment IV, often marginal.
Operative Management
Operative intervention for liver hemangiomas remains a controversial topic. Previous studies from major hepatobiliary centres have proposed varying indications for a hemangioma resection. Findings from the present study demonstrate that operative management of symptomatic hemangiomas remains an effective therapy and can be performed with low morbidity to the patient. However, aside from abdominal symptoms, prophylactic resections in the setting of hemangioma enlargement, size, or patient anxiety is not advised as the risk of developing life-threatening associated complications is rare.
Established Complications. In the minority of cases that present as a surgical emergency due to haemorrhage, rupture, thrombosis and infarction, surgical management may be the only appropriate course of action. There is also a role for the elective surgical management of giant haemangiomata, albeit in a highly selected group of patients. As demonstrated by the data presented above, an operative approach with the objective of preventing future complications of giant haemangiomata is less easy to justify.
Diagnostic Uncertainty. Despite improvements in non-invasive imaging technology, cases of diagnostic uncertainty continue to pose a challenge. In situations where it is not possible to exclude malignancy, surgical intervention by formal liver resection may be indicated. In almost all situations, the use of percutaneous liver biopsy for the differentiation of giant haemangiomata from malignant liver lesions cannot be justified. The risks of haemorrhage as a result of biopsying a giant haemangioma are appreciable and, together with the risks of needle track seeding and intra-abdominal dissemination of a potentially curable malignancy, mean that biopsy in this setting must be avoided.
Incapacitating Symptoms. Having taken all possible steps to ensure that symptoms are attributable to the haemangioma, surgical resection may be justified on grounds of intractable symptoms. Patients with clearly defined abdominal compressive symptoms may be more likely to derive benefit from surgery than patients with non-specific abdominal discomfort, although this is not backed up by a meaningful body of evidence. Management of this group of patients is, by necessity, highly individualised. Despite apparently satisfactory surgical management, symptoms persist in approximately 25% of patients following resection of seemingly symptomatic haemangiomata.
While most people with HH show no sign or symptom, and most HH are non-progressing and do not require treatment, there is a small number of cases with rapid volumetric growth or complications, which prompt for appropriate therapy. The results of clinical and laboratory investigations to date, mostly for imaging techniques, have demonstrated that for small HH, regular follow-up is enough. For cavernous HH, the evolution is unpredictable and often unfavorable, with serious complications requiring particular surgical expertise in difficult cases. Hepatic hemangiomas require a careful diagnosis to differentiate from other focal hepatic lesions, co-occurring diagnoses are also possible.
Surgical management of GASTRIC CANCER
Laparoscopic versus Open gastrectomy
Surgery is the only curative therapy for gastric cancer but most operable gastric cancer presents in a locally advanced stage characterized by tumor infiltration of the serosa or the presence of regional lymph node metastases. Surgery alone is no longer the standard treatment for locally advanced gastric cancer as the prognosis is markedly improved by perioperative chemotherapy. The decisive factor for optimum treatment is the multidisciplinary team specialized in gastric cancer. However, despite multimodal therapy and adequate surgery only 30% of gastric cancer patients are alive at 3 years.
Principles
The same principles that govern open surgery is applied to laparoscopic surgery. To ensure the same effectiveness of laparoscopic gastrectomy (LG) as conventional open gastrectomy, all the basic principles such as properly selected patients, sufficient surgical margins, standardized D2 lymphadenectomy, no-touch technique, etc., should be followed.
Indications
LG may be considered as a safe procedure with better short-term and comparable long-term oncological results compared with open gastrectomy. In addition, there is HRQL advantages to minimal access surgery. There is a general agreement that a laparoscopic approach to the treatment of gastric cancer should be chosen only by surgeons already highly skilled in gastric surgery and other advanced laparoscopic interventions. Furthermore, the first procedures should be carried out during a tutoring program. Diagnostic laparoscopy is strongly recommended as the first step of laparoscopic as well as open gastrectomies. The advantage of early recovery because of reduced surgical trauma would allow earlier commencement of adjuvant chemotherapy and the decreased hospital stay and early return to work may offset the financial costs of laparoscopic surgery.
The first description of LG was given by Kitano, Korea in 1994 and was initially indicated only for early gastric cancer patients with a low-risk lymph node metastasis. As laparoscopic experience has accumulated, the indications for LG have been broadened to patients with advanced gastric cancer. However, the role of LG remains controversial, because studies of the long-term outcomes of LG are insufficient. The Japanese Gastric Cancer Association guidelines in 2004 suggested endoscopic mucosal resection or endoscopic submucosal dissection for stage 1a (cT1N0M0) diagnosis; patients with stage 1b (cT1N1M0) and cT2N0M0) were referred for LG. Totally laparoscopic D2 radical distal gastrectomy using Billroth II anastomosis with laparoscopic linear staplers for early gastric cancer is considered to be safe and feasible. Laparoscopy-assisted total gastrectomy shows better short-term outcomes compared with open total gastrectomy in eligible patients with gastric cancer.
There was a significant reduction of intraoperative blood loss, a reduced risk of postoperative complications, and a shorter hospital stay. Western patients are relatively obese and there is an increased risk of bleeding if lymphadenectomy is performed. LG is technically difficult in the obese than in the normal weight due to reduced visibility, difficulty retracting tissues, dissection plane hindered by adipose tissue, and difficulty with anastomosis. Open gastrectomy is thus preferable for the obese. However, obesity is not a risk factor for survival of patients but it is independently predictive of postoperative complications. Careful approach is being needed, especially for male patients with high body mass index.
Robotic surgery
Robotic surgery will become an additional option in minimally invasive surgery. The importance of performing effective extended lymph node dissection may provide the advantage of using robotic systems. Such developments will improve the quality of life of patients following gastric cancer surgery. A multicenter study with a large number of patients is needed to compare the safety, efficacy, value (efficacy/cost ratio) as well as the long-term outcomes of robotic surgery, traditional laparoscopy, and the open approach.
Classroom: Surgical Management of Gastric Cancer
Principles of Surgical Resection of Hepatocellular Carcinoma
INTRODUCTION
There has been significant improvement in the perioperative results following liver resection, mainly due to techniques that help reduce blood loss during the operation. Extent of liver resection required in HCC for optimal oncologic results is still controversial. On this basis, the rationale for anatomically removing the entire segment or lobe bearing the tumor, would be to remove undetectable tumor metastases along with the primary tumor.
SIZE OF TUMOR VERSUS TUMOR FREE-MARGIN
Several retrospective studies and meta-analyses have shown that anatomical resections are safe in patients with HCC and liver dysfunction, and may offer a survival benefit. It should be noted, that most studies are biased, as non-anatomical resections are more commonly performed in patients with more advanced liver disease, which affects both recurrence and survival. It therefore remains unclear whether anatomical resections have a true long-term survival benefit in patients with HCC. Some authors have suggested that anatomical resections may provide a survival benefit in tumors between 2 and 5 cm. The rational is that smaller tumors rarely involve portal structures, and in larger tumors presence of macrovascular invasion and satellite nodules would offset the effect of aggressive surgical approach. Another important predictor of local recurrence is margin status. Generally, a tumor-free margin of 1 cm is considered necessary for optimal oncologic results. A prospective randomized trial on 169 patients with solitary HCC demonstrated that a resection margin aiming at 2 cm, safely decreased recurrence rate and improved long-term survival, when compared to a resection margin aiming at 1 cm. Therefore, wide resection margins of 2 cm is recommended, provided patient safety is not compromised.
THECNICAL ASPECTS
Intraoperative ultrasound (IOUS) is an extremely important tool when performing liver resections, specifically for patients with HCC and compromised liver function. IOUS allows for localization of the primary tumor, detection of additional tumors, satellite nodules, tumor thrombus, and define relationship with bilio-vascular structures within the liver. Finally, intraoperative US-guided injection of dye, such as methylene-blue, to portal branches can clearly define the margins of the segment supplied by the portal branch and facilitate safe anatomical resection.
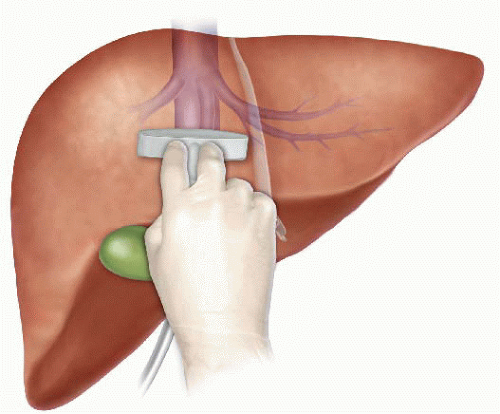
The anterior approach to liver resection is a technique aimed at limiting tumor manipulation to avoid tumoral dissemination, decrease potential for blood loss caused by avulsion of hepatic veins, and decrease ischemia of the remnant liver caused by rotation of the hepatoduodenal ligament. This technique is described for large HCCs located in the right lobe, and was shown in a prospective, randomized trial to reduce frequency of massive bleeding, number of patients requiring blood transfusions, and improve overall survival in this setting. This approach can be challenging, and can be facilitated by the use of the hanging maneuver.

Multiple studies have demonstrated that blood loss and blood transfusion administration are significantly associated with both short-term perioperative, and long-term oncological results in patients undergoing resection for HCC. This has led surgeons to focus on limiting operative blood loss as a major objective in liver resection. Transfusion rates of <20 % are expected in most experienced liver surgery centers. Inflow occlusion, by the use of the Pringle Maneuver represents the most commonly performed method to limit blood loss. Cirrhotic patients can tolerate total clamping time of up to 90 min, and the benefit of reduced blood loss outweighs the risks of inflow occlusion, as long as ischemia periods of 15 min are separated by at least 5 min of reperfusion. Total ischemia time of above 120 min may be associated with postoperative liver dysfunction. Additional techniques aimed at reducing blood loss include total vascular isolation, by occluding the inferior vena cava (IVC) above and below the liver, however, the hemodynamic results of IVC occlusion may be significant, and this technique has a role mainly in tumors that are adjacent to the IVC or hepatic veins.

Anesthesiologists need to assure central venous pressure is low (below 5 mmHg) by limiting fluid administration, and use of diuretics, even at the expense 470 N. Lubezky et al. of low systemic pressure and use of inotropes. After completion of the resection, large amount of crystalloids can be administered to replenish losses during parenchymal dissection.
LAPAROSCOPIC RESECTIONS
Laparoscopic liver resections were shown to provide benefits of reduced surgical trauma, including a reduction in postoperative pain, incision-related morbidity, and shorten hospital stay. Some studies have demonstrated reduced operative bleeding with laparoscopy, attributed to the increased intra-abdominal pressure which reduces bleeding from the low-pressured hepatic veins. Additional potential benefits include a decrease in postoperative ascites and ascites-related wound complications, and fewer postoperative adhesions, which may be important in patients undergoing salvage liver transplantation. There has been a delay with the use of laparoscopy in the setting of liver cirrhosis, due to difficulties with hemostasis in the resection planes, and concerns for possible reduction of portal flow secondary to increased intraabdominal pressure. However, several recent studies have suggested that laparoscopic resection of HCC in patients with cirrhosis is safe and provides improved outcomes when compared to open resections.

Resections of small HCCs in anterior or left lateral segments are most amenable for laparoscopic resections. Larger resections, and resection of posterior-sector tumors are more challenging and should only be performed by very experienced surgeons. Long-term oncological outcomes of laparoscopic resections was shown to be equivalent to open resections on retrospective studies , but prospective studies are needed to confirm these findings. In recent years, robotic-assisted liver resections are being explored. Feasibility and safety of robotic-assisted surgery for HCC has been demonstrated in small non-randomized studies, but more experience is needed, and long-term oncologic results need to be studied, before widespread use of this technique will be recommended.
ALPPS: Associating Liver Partition with Portal vein ligation for Staged hepatectomy
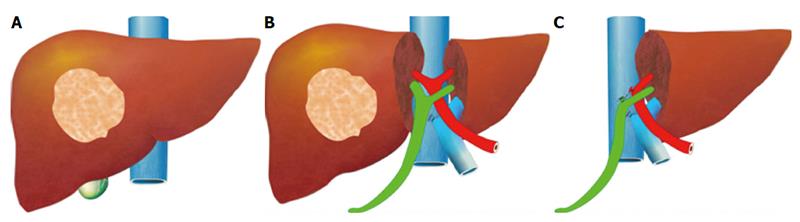
The pre-operative options for inducing atrophy of the resected part and hypertrophy of the FLR, mainly PVE, were described earlier. Associating Liver Partition with Portal vein ligation for Staged hepatectomy (ALPPS) is another surgical option aimed to induce rapid hypertrophy of the FLR in patients with HCC. This technique involves a 2-stage procedure. In the first stage splitting of the liver along the resection plane and ligation of the portal vein is performed, and in the second stage, performed at least 2 weeks following the first stage, completion of the resection is performed. Patient safety is a major concern, and some studies have reported increased morbidity and mortality with the procedure. Few reports exist of this procedure in the setting of liver cirrhosis. Currently, the role of ALPPS in the setting of HCC and liver dysfunction needs to be better delineated before more widespread use is recommended.
Anatomia Cirúrgica Hepática
O Mapa Fundamental para Ressecções e Transplantes
Autor: Prof. Dr. Ozimo Gama
Categoria: Cirurgia Hepatobiliar / Anatomia Aplicada / Transplante Hepático Tempo de Leitura: 12 minutos
“Um bom conhecimento da anatomia do fígado é um pré-requisito para a cirurgia moderna do fígado.” — H. Bismuth
Introdução
O fígado, o maior órgão sólido do corpo humano (representando 2-3% do peso corporal), é uma estrutura de complexidade arquitetônica fascinante. Para o cirurgião geral, e imperativamente para o cirurgião hepatobiliar, o domínio da anatomia hepática transcende a memorização de nomes; trata-se de compreender as relações tridimensionais que ditam a segurança de uma hepatectomia e o sucesso de um transplante. Neste artigo, dissecaremos a anatomia hepática sob uma ótica cirúrgica, indo além da morfologia externa para explorar a segmentação funcional e as nuances vasculares vitais para a prática operatória de excelência.
1. Meios de Fixação e Mobilização Cirúrgica
O fígado é envolto pela cápsula de Glisson e peritônio, exceto na “área nua” diafragmática e no hilo. Seus ligamentos não são apenas estruturas de sustentação, mas marcos anatômicos cruciais para a mobilização segura do órgão:
-
Ligamentos Coronários e Triangulares: A mobilização destes permite a exposição da veia cava inferior (VCI) e das veias hepáticas.
-
Ligamento Venoso (Arantius): Remanescente do ducto venoso fetal. Sua dissecção é uma manobra chave. Ao isolá-lo, o cirurgião ganha acesso ao tronco das veias hepáticas esquerda e média, facilitando o controle vascular em hepatectomias esquerdas ou transplantes.
-
Ligamento Hepatocaval (Makuuchi): Uma estrutura fibrosa (por vezes contendo parênquima) que fixa o lobo caudado à veia cava. Sua divisão cuidadosa é obrigatória para expor a veia hepática direita e para a mobilização completa do lobo direito em transplantes intervivos.
2. A Revolução de Couinaud
A anatomia clássica, que dividia o fígado em lobos direito, esquerdo, quadrado e caudado baseada apenas em marcos externos (como o ligamento falciforme), é insuficiente para a cirurgia moderna. A verdadeira divisão funcional segue a Linha de Cantlie, um plano imaginário que vai do leito da vesícula biliar à veia cava inferior. Esta linha divide o fígado em metades funcionalmente independentes (Direita e Esquerda), cada uma com sua própria irrigação arterial, portal e drenagem biliar.
Adotamos a Segmentação de Couinaud (1954), que organiza o fígado em 8 segmentos baseados na distribuição das veias hepáticas e pedículos portais:
-
Fígado Direito (Setores Anterior e Posterior): Segmentos V, VIII (Anterior) e VI, VII (Posterior).
-
Fígado Esquerdo: Segmentos II, III (Lateral) e IV (Medial).
-
Lobo Caudado (Segmento I): Uma entidade autônoma. Localizado dorsalmente, recebe sangue de ambos os ramos portais (direito e esquerdo) e drena diretamente na VCI através de veias curtas. Esta drenagem direta confere ao caudado uma “proteção” relativa em casos de Síndrome de Budd-Chiari, onde ele frequentemente se hipertrofia.
3. O Hilo Hepático e a Tríade Portal
A dissecção do hilo exige precisão milimétrica, especialmente em transplantes com doador vivo (LDLT). As estruturas da tríade portal seguem uma organização anteroposterior constante que guia o cirurgião:
-
Ducto Biliar: Mais ventral (anterior) e lateral.
-
Artéria Hepática: Medial e na camada intermédia.
-
Veia Porta: A estrutura mais dorsal (posterior).
Variações Vasculares Importantes
-
Artéria Hepática: A anatomia “clássica” (artéria hepática comum saindo do tronco celíaco) está presente em apenas 60% dos casos. Variações críticas incluem a Artéria Hepática Direita Substituída (da Mesentérica Superior), que passa posterior à veia porta, e a Artéria Hepática Esquerda Substituída (da Gástrica Esquerda). O não reconhecimento pode levar à necrose do enxerto ou isquemia biliar.
-
Veia Porta: Variações na bifurcação, como a ausência do tronco principal da veia porta direita (trifurcação), exigem reconstruções complexas em transplantes.
4. Drenagem Venosa: O Escoamento
As três veias hepáticas principais (Direita, Média e Esquerda) correm nas fissuras intersegmentares:
-
Veia Hepática Direita (RHV): Drena o setor posterior. É a maior veia.
-
Veia Hepática Média (MHV): Corre na fissura principal (Linha de Cantlie). Fundamental para a drenagem dos segmentos V e VIII. Em transplantes de lobo direito, a gestão dos tributários da MHV é crítica para evitar congestão do enxerto.
-
Veias Acessórias: Cerca de metade da população possui veias hepáticas acessórias inferiores (drenando os segmentos VI e VII diretamente na cava). Se calibrosas (>5mm), devem ser reimplantadas para garantir a função do enxerto.
5. A Via Biliar e sua Vascularização: O “Tendão de Aquiles”
A anatomia biliar é a mais variável e propensa a complicações.
-
Irrigação Biliar: Diferente do parênquima, os ductos biliares extra-hepáticos são irrigados exclusivamente por um plexo arterial peribiliar (artérias das 3h e 9h), derivado principalmente da artéria hepática direita e retroduodenal.
-
Pérola Cirúrgica: Durante a captação do fígado, a dissecção excessiva do ducto biliar pode desvascularizá-lo, levando a estenoses isquêmicas tardias. Preservar a bainha peribiliar e o tecido hilar é mandatório.
6. A Vesícula Biliar e o Triângulo de Calot
Embora a colecistectomia seja um procedimento comum, ela exige respeito absoluto à anatomia. O Triângulo de Calot (delimitado pelo ducto cístico, ducto hepático comum e borda hepática) é a zona de segurança. A artéria cística deve ser identificada aqui. Variações, como um ducto cístico curto ou inserção no ducto direito, ou uma artéria hepática direita tortuosa (“Hump”) invadindo o triângulo, são armadilhas para o cirurgião desatento.
Conclusão
A cirurgia hepática evoluiu de ressecções em cunha não anatômicas para segmentectomias precisas e transplantes de doadores vivos. Essa evolução foi sustentada por um aprofundamento do conhecimento anatômico. Para o cirurgião em formação, o estudo exaustivo destas estruturas, suas variações e suas relações vasculares não é apenas acadêmico — é a base ética para oferecer segurança e cura aos pacientes portadores de doenças hepatobiliares.
Gostou ❔Nos deixe um comentário ✍️ , compartilhe em suas redes sociais e|ou mande sua dúvida pelo 💬 Chat On-line em nossa DM do Instagram.
Hashtags: #AnatomiaHepatica #CirurgiaDoFigado #TransplanteHepatico #Couinaud #CirurgiaHPB
Pringle Maneuver

After the first major hepatic resection, a left hepatic resection, carried out in 1888 by Carl Langenbuch, it took another 20 years before the first right hepatectomy was described by Walter Wendel in 1911. Three years before, in 1908, Hogarth Pringle provided the first description of a technique of vascular control, the portal triad clamping, nowadays known as the Pringle maneuver. Liver surgery has progressed rapidly since then. Modern surgical concepts and techniques, together with advances in anesthesiological care, intensive care medicine, perioperative imaging, and interventional radiology, together with multimodal oncological concepts, have resulted in fundamental changes. Perioperative outcome has improved significantly, and even major hepatic resections can be performed with morbidity and mortality rates of less than 45% and 4% respectively in highvolume liver surgery centers. Many liver surgeries performed routinely in specialized centers today were considered to be high-risk or nonresectable by most surgeons less than 1–2 decades ago.Interestingly, operative blood loss remains the most important predictor of postoperative morbidity and mortality, and therefore vascular control remains one of the most important aspects in liver surgery.
“Bleeding control is achieved by vascular control and optimized and careful parenchymal transection during liver surgery, and these two concepts are cross-linked.”
First described by Pringle in 1908, it has proven effective in decreasing haemorrhage during the resection of the liver tissue. It is frequently used, and it consists in temporarily occluding the hepatic artery and the portal vein, thus limiting the flow of blood into the liver, although this also results in an increased venous pressure in the mesenteric territory. Hemodynamic repercussion during the PM is rare because it only diminishes the venous return in 15% of cases. The cardiovascular system slightly increases the systemic vascular resistance as a compensatory response, thereby limiting the drop in the arterial pressure. Through the administration of crystalloids, it is possible to maintain hemodynamic stability.

In the 1990s, the PM was used continuously for 45 min and even up to an hour because the depth of the potential damage that could occur due to hepatic ischemia was not yet known. During the PM, the lack of oxygen affects all liver cells, especially Kupffer cells which represent the largest fixed macrophage mass. When these cells are deprived of oxygen, they are an endless source of production of the tumour necrosis factor (TNF) and interleukins 1, 6, 8 and 10. IL 6 has been described as the cytokine that best correlates to postoperative complications. In order to mitigate the effects of continuous PM, intermittent clamping of the portal pedicle has been developed. This consists of occluding the pedicle for 15 min, removing the clamps for 5 min, and then starting the manoeuvre again. This intermittent passage of the hepatic tissue through ischemia and reperfusion shows the development of hepatic tolerance to the lack of oxygen with decreased cell damage. Greater ischemic tolerance to this intermittent manoeuvre increases the total time it can be used.
Recurrence after Repair of Incisional Hernia

The incidence of recurrence in incisional hernia prosthetic surgery is markedly lower than in direct plasties. Indeed after the autoplasties of the preprosthetic period, the recurrence rate ranged from 35% for ventral hernias. Chevrel and Flament, in 1990, reported on 1,033 patients who had undergone laparotomy. The recurrence rate at 10-year follow-up was 14–24% for patients treated without the use of prostheses but only 8.6% for those in whom a prosthesis was implanted. A similar incidence was reported by Chevrel in 1995: 18.3% recurrence without prostheses, 5.5% with prostheses. Likewise, Wantz, in 1991, noted a recurrence rate of 0–18.5% in prosthetic laparo-alloplasties.
At the European Hernia Society (EHS)-GREPA meeting in 1986, the recurrence rate without prostheses was reported to be between 7.2 and 17% whereas in patients who had been treated with a prosthesis the recurrence was between 1 and 5.8%. A case study published by Flament in 1999 showed a 5.6% recurrence rate for operations with prostheses placed behind the muscles and in front of the fascia, and a 3.6% of such figure consisted of a small-sized lateroprosthetic recurrence. These rates were in contrast to the 26.8% recurrence reported by other surgeons for operations without prostheses.
Studies of recurrence are, of course, influenced by the size of the initial defect and the length of follow-up. Nevertheless, it is beyond dispute that the use of prostheses is associated with a lower rate of recurrence independent of the nature of the incisional hernia. The factors that lead to relapse are recognisable in the original features of the ventral hernia, i.e. combined musculo-aponeurotic parietal involvement, septic complications in the first operation, the nature and appropriateness of treatment, the kind of prosthesis and its position. Also important is whether the surgery was an emergency case and the relation to occlusive phenomena, visceral damage
and whether these problems were addressed at the same time.
Obesity is also an important risk factor for recurrence. In addition to its association with a higher surgical complications rate, related to the high intraabdominal pressure, there are deficits in wound cicatrisation as well as respiratory and metabolic pathologies. In such patients, the laparoscopic approach is very useful to significantly reduce the onset of general and wall complications, and the data concerning recurrence are encouraging, ranging between 1 and 9% in the largest laparoscopic case studies. The important multicentric study of Heniford et al., in 2000, reported a recurrence rate of 3.4% after 23 months. In 2003, the same author, in a study with an average follow-up of 20 months (range 1–96) showed a recurrence rate of 4.7% for different, identifiable causes: intestinal iatrogenic injuries and mesh infection with its removal, insufficient fixation of the prosthesis and abdominal trauma in the first postoperative period.
The incidence of recurrence after laparoscopic treatment may also be related to general patient factors and to the onset of local complications, mistakes in opting for laparoscopic treatment and deficits in implanting and fixing the prosthesis. With respect to the latter, it is very important to allow a large overlap compared to the diameter of the defect. Long-term data analysis, with large case studies, is still needed to obtain detailed information about recurrence, and this is particularly true in the assessment of relatively new techniques.
Management of gallbladder cancer

Gallbladder cancer is uncommon disease, although it is not rare. Indeed, gallbladder cancer is the fifth most common gastrointestinal cancer and the most common biliary tract cancer in the United States. The incidence is 1.2 per 100,000 persons per year. It has historically been considered as an incu-rable malignancy with a dismal prognosis due to its propensity for early in-vasion to liver and dissemination to lymph nodes and peritoneal surfaces. Patients with gallbladder cancer usually present in one of three ways: (1) advanced unresectable cancer; (2) detection of suspicious lesion preoperatively and resectable after staging work-up; (3) incidental finding of cancer during or after cholecystectomy for benign disease.
SURGICAL MANAGEMENT
Although, many studies have suggested improved survival in patients with early gallbladder cancer with radical surgery including en bloc resection of gallbladder fossa and regional lymphadenectomy, its role for those with advanced gallbladder cancer remains controversial. First, patients with more advanced disease often require more extensive resections than early stage tumors, and operative morbidity and mortality rates are higher. Second, the long-term outcomes after resection, in general, tend to be poorer; long-term survival after radical surgery has been reported only for patients with limited local and lymph node spread. Therefore, the indication of radical surgery should be limited to well-selected patients based on thorough preoperative and intra-operative staging and the extent of surgery should be determined based on the area of tumor involvement.
Surgical resection is warranted only for those who with locoregional disease without distant spread. Because of the limited sensitivity of current imaging modalities to detect metastatic lesions of gallbladder cancer, staging laparoscopy prior to proceeding to laparotomy is very useful to assess the
abdomen for evidence of discontinuous liver disease or peritoneal metastasis and to avoid unnecessary laparotomy. Weber et al. reported that 48% of patients with potentially resectable gallbladder cancer on preoperative imaging work-up were spared laparotomy by discovering unresectable disease by laparoscopy. Laparoscopic cholecystectomy should be avoided when a preoperative cancer is suspected because of the risk of violation of the plane between tumor and liver and the risk of port site seeding.
The goal of resection should always be complete extirpation with microscopic negative margins. Tumors beyond T2 are not cured by simple cholecystectomy and as with most of early gallbladder cancer, hepatic resection is always required. The extent of liver resection required depends upon whether involvement of major hepatic vessels, varies from segmental resection of segments IVb and V, at minimum to formal right hemihepatectomy or even right trisectionectomy. The right portal pedicle is at particular risk for advanced tumor located at the neck of gallbladder, and when such involvement is suspected, right hepatectomy is required. Bile duct resection and reconstruction is also required if tumor involved in bile duct. However, bile duct resection is associated with increased perioperative morbidity and it should be performed only if it is necessary to clear tumor; bile duct resection does not necessarily increase the lymph node yield.
Hepatic Surgery: Portal Vein Embolization

INTRODUCTION
Portal vein Embolizations (PVE) is commonly used in the patients requiring extensive liver resection but have insufficient Future Liver Remanescent (FLR) volume on preoperative testing. The procedure involves occluding portal venous flow to the side of the liver with the lesion thereby redirecting portal flow to the contralateral side, in an attempt to cause hypertrophy and increase the volume of the FLR prior to hepatectomy.
PVE was first described by Kinoshita and later reported by Makuuchi as a technique to facilitate hepatic resection of hilar cholangiocarcinoma. The technique is now widely used by surgeons all over the world to optimize FLR volume before major liver resections.
PHYSIOPATHOLOGY
PVE works because the extrahepatic factors that induce liver hypertrophy are carried primarily by the portal vein and not the hepatic artery. The increase in FLR size seen after PVE is due to both clonal expansion and cellular hypertrophy, and the extent of post-embolization liver growth is generally proportional to the degree of portal flow diversion. The mechanism of liver regeneration after PVE is a complex phenomenon and is not fully understood. Although the exact trigger of liver regeneration remains unknown, several studies have identified periportal inflammation in the embolized liver as an important predictor of liver regeneration.

THECNICAL ASPECTS
PVE is technically feasible in 99% of the patients with low risk of complications. Studies have shown the FLR to increase by a median of 40–62% after a median of 34–37 days after PVE, and 72.2–80% of the patients are able to undergo resection as planned. It is generally indicated for patients being considered for right or extended right hepatectomy in the setting of a relatively small FLR. It is rarely required before extended left hepatectomy or left trisectionectomy, since the right posterior section (segments 6 and 7) comprises about 30% of total liver volume.
PVE is usually performed through percutaneous transhepatic access to the portal venous system, but there is considerable variability in technique between centers. The access route can be ipsilateral (portal access at the same side being resected) with retrograde embolization or contralateral (portal access through FLR) with antegrade embolization. The type of approach selected depends on a number of factors including operator preference, anatomic variability, type of resection planned, extent of embolization, and type of embolic agent used. Many authors prefer ipsilateral approach especially for right-sided tumors as this technique allows easy catheterization of segment 4 branches when they must be embolized and also minimizes the theoretic risk of injuring the FLR vasculature or bile ducts through a contralateral approach and potentially making a patient ineligible for surgery.
However, majority of the studies on contralateral PVE show it to be a safe technique with low complication rate. Di Stefano et al. reported a large series of contralateral PVE in 188 patients and described 12 complications (6.4%) only 6 of which could be related to access route and none precluded liver resection. Site of portal vein access can also change depending on the choice of embolic material selected which can include glue, Gelfoam, n-butyl-cyanoacrylate (NBC), different types and sizes of beads, alcohol, and nitinol plus. All agents have similar efficacy and there are no official recommendations for a particular type of agent.
RESULTS
Proponents of PVE believe that there should be very little or no tumor progression during the 4–6 week wait period for regeneration after PVE. Rapid growth of the FLR can be expected within the first 3–4 weeks after PVE and can continue till 6–8 weeks. Results from multiple studies suggest that 8–30% hypertrophy over 2–6 weeks can be expected with slower rates in cirrhotic patients. Most studies comparing outcomes after major hepatectomy with and without preoperative PVE report superior outcomes with PVE. Farges et al. demonstrated significantly less risk of postoperative complications, duration of intensive care unit, and hospital stay in patients with cirrhosis who underwent right hepatectomy after PVE compared to those who did not have preoperative PVE. The authors also reported no benefit of PVE in patients with a normal liver and FLR >30%. Abulkhir et al. reported results from a meta-analysis of 1088 patients undergoing PVE and showed a markedly lower incidence of Post Hepatectomy Liver Failure (PHLF) and death compared to series reporting outcomes after major hepatectomy in patients who did not undergo PVE. All patients had FLR volume increase, and 85% went on to have liver resection after PVE with a PHLF incidence of 2.5% and a surgical mortality of 0.8%. Several studies looking at the effect of systemic neoadjuvant chemotherapy on the degree of hypertrophy after PVE show no significant impact on liver regeneration and growth.
VOLUMETRIC RESPONSE

The volumetric response to PVE is also a very important factor in understanding the regenerative capacity of a patient’s liver and when used together with FLR volume can help identify patients at risk of poor postsurgical outcome. Ribero et al. demonstrated that the risk of PHLF was significantly higher not only in patients with FLR ≤ 20% but also in patients with normal liver who demonstrated ≤5% of FLR hypertrophy after PVE. The authors concluded that the degree of hypertrophy >10% in patients with severe underlying liver disease and >5% in patients with normal liver predicts a low risk of PHLF and post-resection mortality. Many authors do not routinely offer resection to patients with borderline FLR who demonstrate ≤5% hypertrophy after PVE.
Predicting LIVER REMNANT Function
Careful analysis of outcome based on liver remnant volume stratified by underlying liver disease has led to recommendations regarding the safe limits of resection. The liver remnant to be left after resection is termed the future liver remnant (FLR). For patients with normal underlying liver, complications, extended hospital stay, admission to the intensive care unit, and hepatic insufficiency are rare when the standardized FLR is >20% of the TLV. For patients with tumor-related cholestasis or marked underlying liver disease, a 40% liver remnant is necessary to avoid cholestasis, fluid retention, and liver failure. Among patients who have been treated with preoperative systemic chemotherapy for more than 12 weeks, FLR >30% reduces the rate of postoperative liver insufficiency and subsequent mortality.
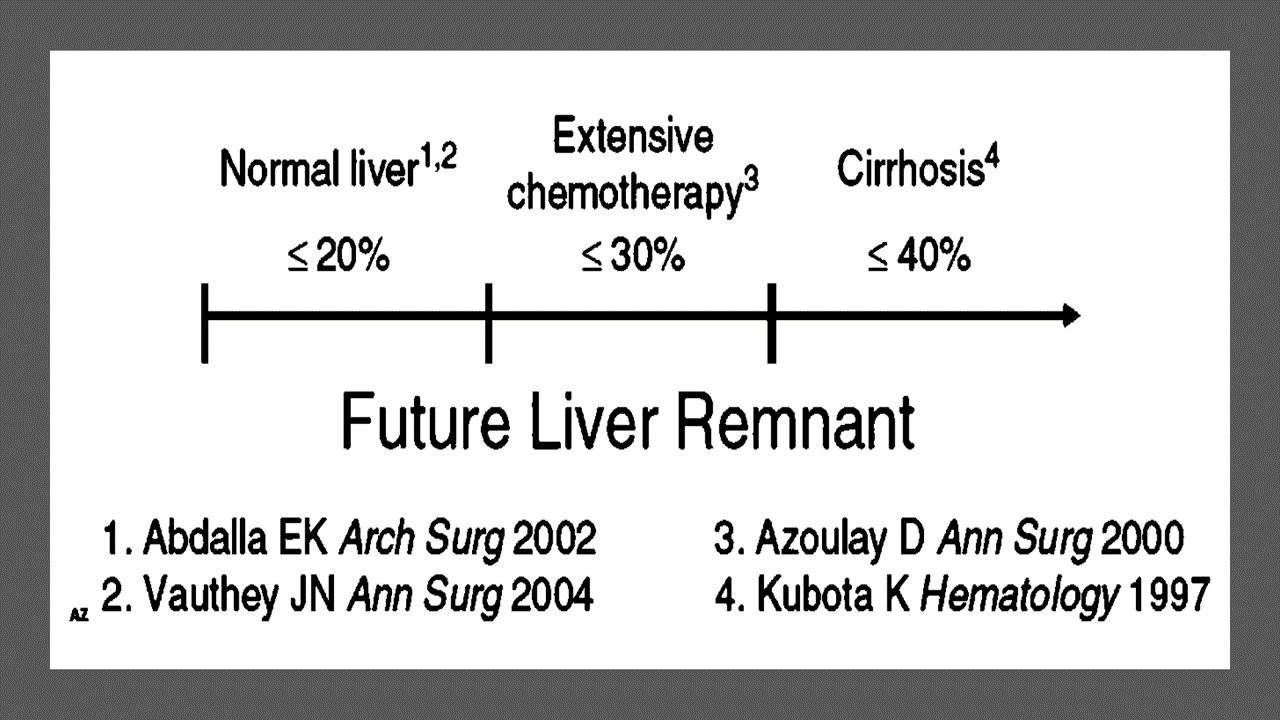
When the liver remnant is normal or has only mild disease, the volume of liver remnant can be measured directly and accurately with threedimensional computed tomography (CT) volumetry. However, inaccuracy may arise because the liver to be resected is often diseased, particularly in patients with cirrhosis or biliary obstruction. When multiple or large tumors occupy a large volume of the liver to be resected, subtracting tumor volumes from liver volume further decreases accuracy of CT volumetry. The calculated TLV, which has been derived from the association between body surface area (BSA) and liver size, provides a standard estimate of the TLV. The following formula is used:
TLV (cm3) = –794.41 + 1267.28 × BSA (square meters)
Thus, the standardized FLR (sFLR) volume calculation uses the measured FLR volume from CT volumetry as the numerator and the calculated TLV as the denominator: Standardized FLR (sFLR) = measured FLR volume/TLV Calculating the standardized TLV corrects the actual liver volume to the individual patient’s size and provides an individualized estimate of that patient’s postresection liver function. In the event of an inadequate FLR prior to major hepatectomy, preoperative liver preparation may include portal vein embolization (PVE).
Classroom: Principles of Hepatic Surgery
Videos of Surgical Procedures

This page provides links to prerecorded webcasts of surgical procedures. These are actual operations performed at medical centers in the Brazil. Please note that you cannot send in questions by email, though the webcast may say that you can, because you are not seeing these videos live. The videos open in a second window. If you have a pop-up blocker, you will need to disable it to view the programs.
Videos of Surgical Procedures
Surgical Management of Cholangiocarcinoma

Cholangiocarcinoma (CCA) is a rare but lethal cancer arising from the bile duct epithelium. As a whole, CCA accounts for approximately 3 % of all gastrointestinal cancers. It is an aggressive disease with a high mortality rate. Unfortunately, a significant proportion of patients with CCA present with either unresectable or metastatic disease. In a retrospective review of 225 patients with hilar cholangiocarcinoma, Jarnagin et al. reported that 29 % of patients had either unresectable disease were unfit for surgery. Curative resection offers the best chance for longterm survival. Whereas palliation with surgical bypass was once the preferred surgical procedure even for resectable disease, aggressive surgical resection is now the standard.
Classroom: Surgical Management of Cholangiocarcinoma
Strangulation in GROIN HERNIAS
Importance
Declining Mortality Rates
In both the UK and the USA, the annual death rate due to inguinal and femoral hernias has significantly decreased over the past two to three decades. In the UK, deaths from these hernias declined by 22% to 55% between 1975 and 1990. Similarly, in the USA, the annual deaths per 100,000 population for patients with hernia and intestinal obstruction decreased from 5.1 in 1968 to 3.0 in 1988. For patients with obstructed inguinal hernias, 88% underwent surgery, with a remarkably low mortality rate of 0.05%. These improvements suggest that elective groin hernia surgery has played a crucial role in reducing overall mortality rates.
Elective Surgery and Strangulation Rates
Supporting this observation, the USA has lower rates of strangulation compared to the UK, possibly due to the threefold higher rate of elective hernia surgeries in the USA. Nevertheless, statistics indicate that the rate of elective hernia surgeries in the USA per 100,000 population decreased from 358 to 220 between 1975 and 1990, although this may be an artifact of data collection rather than a genuine decline.
Mortality Analysis from UK and Denmark Studies
During 1991–1992, the UK National Confidential Enquiry Into Perioperative Deaths investigated 210 deaths following inguinal hernia repair and 120 deaths following femoral hernia repair. This inquiry, which focuses on the quality of surgery, anesthesia, and perioperative care, found that many patients were elderly (45 were aged 80–89 years) and significantly infirm; 24 were ASA grade III and 21 ASA grade IV. The majority of postoperative mortality was attributed to preexisting cardiorespiratory issues.
A nationwide study in Denmark of 158 patients who died after acute groin hernia repair by Kjaergaard et al. also found that these patients were old (median age 83 years) and frail (>80% with significant comorbidity), with frequent delays in diagnosis and treatment. These findings highlight the need for high-quality care by experienced surgeons and anesthetists, especially for patients with high ASA grades.
Postoperative Care Recommendations
Postoperative care for these patients should occur in a high-dependency unit or intensive therapy unit. This might necessitate transferring selected patients to appropriate hospitals and facilities. Decisions about interventional surgery should be made in consultation with the relatives of extremely elderly, frail, or moribund patients, adopting a humane approach that may rule out surgery.
Emergency Admissions and Prioritization
Forty percent of patients with femoral hernias are admitted as emergency cases with strangulation or incarceration, while only 3% of patients with direct inguinal hernias present with strangulation. This disparity has implications for prioritizing patients on waiting lists when these hernias present electively in outpatient clinics.
Risk of Strangulation
A groin hernia is at its greatest risk of strangulation within three months of onset. For inguinal hernias, the cumulative probability of strangulation is 2.8% at three months after presentation, rising to 4.5% after two years. The risk is much higher for femoral hernias, with a 22% probability of strangulation at three months, rising to 45% at 21 months. Right-sided hernias have a higher strangulation rate than left-sided hernias, potentially due to anatomical differences in mesenteric attachment. The decline in hernia-related mortality in both the UK and USA underscores the importance of elective hernia surgery. Ensuring timely surgery, especially for high-risk femoral hernias, and providing high-quality perioperative care for elderly and frail patients are crucial steps in further reducing mortality and improving patient outcomes.
Evidence-Based Medicine
In a randomized trial, evaluating an expectative approach to minimally symptomatic inguinal hernias, Fitzgibbons et al. in the group of patients randomized to watchful waiting found a risk of an acute hernia episode of 1.8 in 1,000 patient years. In another trial, O’Dwyer and colleagues, randomizing patients with painless inguinal hernias to observation or operation, found two acute episodes in 80 patients randomized to observation. In both studies, a large percentage of patients randomized to nonoperative care were eventually operated due to symptoms. Neuhauser, who studied a population in Columbia where elective herniorrhaphy was virtually unobtainable, found an annual rate of strangulation of 0.29% for inguinal hernias.
Management of Strangulation
The diagnosis of hernias is primarily based on clinical symptoms and signs, supplemented by imaging studies when necessary. Pain at the hernia site is a constant symptom. In cases of obstruction with intestinal strangulation, patients may present with colicky abdominal pain, distension, vomiting, and constipation. Physical examination may reveal signs of dehydration, with or without central nervous system depression, especially in elderly patients with uremia, along with abdominal signs of intestinal obstruction.
Femoral hernias can be easily missed, particularly in obese women, making a thorough physical examination essential for an accurate diagnosis. However, physical examination alone is often insufficient to confirm the presence of a strangulated femoral hernia versus lymphadenopathy or a lymph node abscess. In such cases, urgent radiographic studies, such as ultrasound or CT scan, may be necessary.
The choice of incision depends on the type of hernia if the diagnosis is clear. When there is doubt, a half Pfannenstiel incision, 2 cm above the pubic ramus extending laterally, provides adequate access to all types of femoral or inguinal hernias. The fundus of the hernia sac is exposed, and an incision is made to assess the viability of its contents. If nonviability is detected, the transverse incision should be converted into a laparotomy incision, followed by the release of the constricting hernia ring, reduction of the sac’s contents, resection, and reanastomosis. Precautions must be taken to avoid contamination of the general peritoneal cavity by gangrenous bowel or intestinal contents.
In most cases, once the constriction of the hernia ring is released, circulation to the intestine is restored, and viability returns. The intestine that initially appears dusky or non-peristaltic may regain color with a short period of warming with damp packs. If viability is doubtful, resection should be performed. Resection rates are highest for femoral or recurrent inguinal hernias and lowest for simple inguinal hernias. Other organs, such as the bladder or omentum, should be resected as needed.
After peritoneal lavage and formal closure of the laparotomy incision, specific repair of the hernia should be performed. Prosthetic mesh should not be used in a contaminated operative field due to the high risk of wound infection. Hernia repair should follow the general principles of elective hernia repair. It is important to remember that in this predominantly frail and elderly patient group with a high postoperative mortality risk, the primary objective of the operation is to stop the vicious cycle of strangulation, with hernia repair being a secondary objective.
Key Point
The risk of an acute groin hernia episode is of particular relevance, when discussing indication for operation of painless or minimally symptomatic hernias. A sensible approach in groin hernias would be, in accordance with the guidelines from the European Hernia Society to advise a male patient, that the risk of an acute operation, with an easily reducible (“disappears when lying down”) inguinal hernia with little or no symptoms, is low and that the indication for operation in this instance is not absolute, but also inform, that usually the hernia after some time will cause symptoms, eventually leading to an operation. In contrast, female patients with a groin hernia, due to the high frequency of femoral hernias and a relatively high risk of acute hernia episodes, should usually be recommended an operation.
Abdominal Surgical Anatomy
The abdomen is the lower part of the trunk below the diaphragm. Its walls surround a large cavity called the abdominal cavity. The abdominal cavity is much more extensive than what it appears from the outside. It extends upward deep to the costal margin up to the diaphragm and downward within the bony pelvis. Thus, a considerable part of the abdominal cavity is overlapped by the lower part of the thoracic cage above and by the bony pelvis below. The abdominal cavity is subdivided by the plane of the pelvic inlet into a larger upper part, i.e., the abdominal cavity proper, and a smaller lower part, i.e., the pelvic cavity. Clinically the importance of the abdomen is manifold. To the physician, the physical examination of the patient is never complete until he/she thoroughly examines the abdomen. To the surgeon, the abdomen remains an enigma because in number of cases the cause of abdominal pain and nature of abdominal lump remains inconclusive even after all possible investigations. To summarize, many branches of medicine such as general surgery and gastroenterology are all confined to the abdomen.
Classroom: Abdominal Surgical Anatomy
Minimally Invasive Approach to Choledocholithiasis
Introduction
The incidence of choledocholithiasis in patients undergoing cholecystectomy is estimated to be 10 %. The presence of common bile duct stones is associated with several known complications including cholangitis, gallstone pancreatitis, obstructive jaundice, and hepatic abscess. Making the diagnosis early and prompt management is crucial. Traditionally, when choledocholithiasis is identified with intraoperative cholangiography during the cholecystectomy, it has been managed surgically by open choledochotomy and place- ment of a T-tube. This open surgical approach has a morbidity rate of 10–15 %, mortality rate of <1 %, with a <6 % incidence of retained stones. Patients who fail endoscopic retrieval of CBD stones, as well as cases in which an endoscopic approach is not appropriate, should be explored surgically.
Clinical Manifestation
Acute obstruction of the bile duct by a stone causes a rapid distension of the biliary tree and activation of local pain fibers. Pain is the most common presenting symptom for choledocholithiasis and is localized to either the right upper quadrant or to the epigastrium. The obstruction will also cause bile stasis which is a risk factor for bacterial over- growth. The bacteria may originate from the duodenum or the stone itself. The combination of biliary obstruction and colo- nization of the biliary tree will lead to the development of fevers, the second most common presenting symptom of cho- ledocholithiasis. Biliary obstruction, if unrelieved, will lead to jaundice. When these three symptoms (pain, fever, and jaundice) are found simultaneously, it is known as Charcot’s triad. This triad suggests the diagnosis of acute ascending cholangitis, a potentially life-threatening condition. If not treated promptly, this can lead to hypotension and decreased metal status, both signs of severe sepsis. When combined with Charcot’s triad, this constellation of symptoms is commonly referred to as Reynolds pentad.
Laparoscopic common bile duct exploration
Laparoscopic common bile duct exploration (LCBDE) allows for single stage treatment of gallstone disease, reducing overall hospital stay, improving safety and cost-effectiveness when compared to the two-stage approach of ERCP and laparoscopic cholecystectomy. Bile duct clearance can be confirmed by direct visualization with a choledochoscope. But, before the advent of choledochoscope, bile duct clearance was uncertain, and blind instrumentation of the duct resulted in accentuated edema and inflammation. Due to advancement in instruments, optical magnification, and direct visualization, laparoscopic exploration of the CBD results in fewer traumas to the bile duct. This has led to an increasing tendency to close the duct primarily, reducing the need for placement of T-tubes. Still, laparoscopic bile duct exploration is being done in only a few centers. Apart from the need for special instruments, there is also a significant learning curve to acquire expertise to be able to perform a laparoscopic bile duct surgery.
Morbidity and mortality rates of laparoscopic exploration are comparable to ERCP (2–17 and 1–5 %), and there is no clear difference in primary success rates between the two approaches. However, the endoscopic approach may be preferable for elderly and frail patients, who are at higher risk with surgery. Patients older than 70–80 years of age have a 4–10 % mortality rate with open duct exploration. It may be as high as 20 % in elderly patients undergoing urgent procedures. In comparison, advanced age and comor- bidities do not have a significant impact on overall complication rates for ERCP. A success rate of over 90 % has been reported with laparoscopic CBD exploration. Availability of surgical expertise and appropriate equipment affect the success rate of laparoscopic exploration, as does the size, number of the CBD stones, as well as biliary anatomy. Over the years, laparoscopic exploration has become efficient, safe, and cost effective. Complications include CBD laceration, stricture formation, bile leak, abscess, pancreatitis, and retained stones.
In cases of failure of laparoscopic CBD exploration, a guidewire or stent can be passed through the cystic duct, common bile duct, and through the ampulla into the duodenum followed by cholecystectomy. This makes the identification and cannulation of the ampulla easier during the post- operative ERCP. Laparoscopic common bile duct exploration is traditionally performed through a transcystic or transductal approach. The transcystic approach is appropriate under certain circumstances. These include a small stone (<10 mm) located in the CBD, presence of small common bile duct (<6 mm), or if there is poor access to the common duct. The transductal approach is preferable in cases of large stones, stones in proximal ducts (hepatic ducts), large occluding stones in a large duct, presence of multiple stones, or if the cystic duct is small (<4 mm) or tortuous. Contraindications for laparoscopic approach include lack of training, and severe inflammation in the porta hepatis making the exploration difficult and risky.
Key Points
With advancement in imaging technology, laparoscopic and endoscopic techniques, management of common bile duct stone has changed drasti- cally in recent years. This has made the treatment of this condition safe and more efficient. Many options are now available to manage this condition, and any particular modality for treatment should be chosen carefully based on the patient related factors, institutional protocol, available expertise, resources, and cost-effectiveness.
Classroom: M.I.A. of Choledocholithiasis
Management of Complicated Appendicitis: Open or Laparoscopic Surgery?
Patients with acute appendicitis can present at different stages of the disease process, ranging from mild mucosal inflammation to frank perforation with abscess formation. The reported overall incidence of acute appendicitis varies with age, gender, and geographical differences. Interestingly, while the incidence of non-perforated appendicitis in the United States decreased between 1970 and 2004, no significant decline in the rate of perforated appendicitis was observed despite the increasng use of computed tomography (CT) and fewer negative appendectomies.
Of 32,683 appendectomies sampled from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) hospitals between 2005 and 2008, 5,405 patients (16.5%) had a preoperative diagnosis of acute appendicitis with peritonitis/abscess.
The definition of complicated appendicitis varies slightly in the literature. Clinicopathological diagnoses (gangrenous, perforated, appendiceal abscess/phlegmon) of acute appendicitis are commonly used for its definition. Classically, patients at the extremes of age are more likely to present with complicated appendicitis. Similarly, pre-morbid conditions including diabetes and type of medical insurance are significantly associated with the risk of perforation.
The importance of early appendectomy has also been emphasized to prevent perforation of the appendix and the sub- sequent negative impact on patient outcomes. However, more recent meta-analysis data supports the safety of a relatively short (12–24 h) delay before appendectomy, which was not significantly associated with increased rate of complicated appendicitis. Teixeira et al. also showed that the time to appendectomy was not a significant risk factor for perforated appendicitis but did result in a significantly increased risk of surgical site infection.
The outcome of patients with complicated appendicitis is significantly worse than patients with uncomplicated appendicitis. A population-based study from Sweden showed that, in a risk-adjusted model, patients with perforated appendicitis were 2.34 times more likely to die after appendectomy than non- perforated appendicitis patients. Because of its higher mortality and morbidity in patients with complicated appendicitis, the management of complicated appendicitis has evolved significantly over the last few decades.
Open or Laparoscopic Surgery
Since the first laparoscopic appendectomy was described by Semm in 1983, multiple studies have compared operative time, complication rates, length of hospital stay, hospital cost, and other outcomes between open and laparoscopic appendectomy for acute appendicitis. The most recent Cochrane review included 67 studies showing that laparoscopic appendectomy was associated with a lower incidence of wound infection, reduced postoperative pain, shorter postoperative length of hospital stay, and faster recovery to daily activity. In contrast, reduced risk of intra-abdominal abscesses and shorter operative time were found as the advantages of open appendectomy.
Due to increased surgeon experience in uncomplicated appendicitis, laparoscopic appendectomy is more frequently attempted even in complicated appendicitis cases as an alternative approach to open appendectomy. Although the general surgical steps for complicated appendicitis are similar to those for uncomplicated appendicitis, the laparoscopic procedure can be more technically demanding. Therefore, conversion from laparoscopic appendectomy to open appendectomy can be expected.
Despite these concerns, the laparoscopic approach in patients with com- plicated appendicitis has been proven to be safe and comparable to open appendectomy. Retrospective studies using a large database in the United States uniformly showed more favorable clinical outcomes (mortality, morbidity, length of hospital stay, readmission rate) and hospital costs in patients who underwent laparoscopic appendectomy when compared to open appendectomy. The real risk of developing an intra- abdominal abscess after laparoscopic appendectomy remains unclear. A meta-analysis by Markides et al. found no significant difference in the intra-abdominal abscess rate between laparoscopic and open appendectomy for complicated appendicitis, whereas Ingraham et al. showed a higher likelihood of developing an organ-space surgical site infection in patients undergoing laparoscopic appendectomy.
Non Operative Management of SPLENIC TRAUMA

The spleen, an important component of the reticuloendothelial system in normal adults, is a highly vascular solid organ that arises as a mass of differentiated mesenchymal tissue during early embryonic development. The normal adult spleen weighs between 75 and 100 g and receives an average blood flow of 300 mL/min. It functions as the primary filter of the reticuloendothelial system by sequestering and removing antigens, bacteria, and senescent or damaged cellular elements from the circulation. In addition, the spleen has an important role in humoral immunity because it produces immunoglobulin M and opsonins for the complement activation system.
The increased availability of high-resolution CT scan and advances in arterial angiography and embolization techniques have contributed to the success of nonoperative management of splenic injuries.
The hemodynamically stable patient with blunt splenic trauma can be adequately managed with bed rest, serial abdominal exams, and hemoglobin and hematocrit monitoring. This approach, in combination with occasional angiography, especially for grade III and IV injuries, confers a splenic salvage rate of up to 95%.
In the setting of expectant management, indications for angiography have been delineated by several studies and include the following CT scan features: contrast extravasation, the presence of a pseudoaneurysm, significant hemoperitoneum, high-grade injury, and evidence of a vascular injury. The goal of angiography is to localize bleeding and embolize the source with coils or a gelatin foam product. Embolization can occur either at the main splenic artery just distal to the dorsal pancreatic portion of the vessel—known as proximal embolization—or selectively at the distal branch of the injured vessel. The goal behind the former technique is to decrease the perfusion pressure to the spleen to encourage hemostasis. The disadvantage to this technique is global splenic ischemia, and many have questioned the spleen’s immunocompetence following proximal embolization.
Malhotra et al. examined the effects of angioembolization on splenic function by examining serum levels of a particular T-cell line. T-cell proportions between patients who had undergone splenic embolization with asplenic patients and healthy controls were similar suggesting some degree of splenic immunocompetency was maintained. A Norwegian study comparing blood samples from patients who had undergone angioembolization with healthy controls demonstrated that the study samples had similar levels of pneumococcal immunoglobulins and no Howell-Jolly bodies, suggesting normal splenic function. Although these preliminary studies remain encouraging, there is no definitive evidence that splenic immunocompetency is fully maintained following angio-embolization.
There is no question that advancements in interventional techniques have contributed to the successful nonoperative management of splenic injuries. This has certainly changed the strategy, but it has not completely replaced operative intervention. The challenge now remains predicting those patients who will ultimately require splenectomy.
Many groups have studied potential predictors of nonoperative failure. Earlier studies found that a higher injury grade, increased transfusion requirement, and hypotension on initial presentation consistently predicted failure of nonoperative management. More recent literature reflects the use of advanced imaging techniques for predicting which patients will ultimately require splenectomy. Haan looked at the overall outcomes of patients admitted with blunt splenic trauma and reported several radiographic findings that were prevalent among patients requiring splenectomy after angioembolization:
- contrast extravasation,
- pseudoaneurysm,
- significant hemoperitoneum,
- and arteriovenous fistula.
Among these characteristics, an arteriovenous fistula had the highest rate of nonoperative failure at 40%. Nonradiographic features associated with significant risk of nonoperative failure include age greater than 40, injury severity score of 25 or greater, or presence of large-volume hemoperitoneum.
Aside from radiographic findings, some groups have also examined the mechanism of injury and its association with nonoperative failure. Plurad et al. conducted a retrospective review over a 15-year period and found that patients who were victims of blunt assault were more likely to fail nonoperative management: 36% of these patients required splenectomy versus 11.5% of patients from all other mechanisms combined. These findings suggest that regardless of overall injury severity, individuals who sustain a direct transfer of injury to the left torso are more likely to require splenectomy.
Currently, the accepted standard of care for most splenic trauma is expectant management with close observation. Operative intervention is reserved for the hemodynamically labile patient who shows signs of active hemorrhage and who does not respond appropriately to fluid resuscitation. Although these clinical scenarios seem straightforward, it is often the condition of the patient who falls in between the two ends of the spectrum that can be the most challenging to manage. In the setting of advanced imaging techniques and interventional radiology, the trauma surgeon has more diagnostic information and more treatment options for the patient with splenic trauma.
IPMN Surgical Management

INTRODUCTION
IPMNs were first recognized in 1982 by Ohashi, but the term IPMN was not officially used until 1993. IPMNs are defined in the WHO Classification of Tumors of the Digestive System as an intraductal, grossly visible epithelial neoplasm of mucin-producing cells. Using imaging and histology, IPMNs can be classified into three types based on duct involvement:
1. Main-duct IPMN (approximately 25% of IPMNs): Segmental or diffuse dilation of the main pancreatic duct (>5 mm) in the absence of other causes of ductal obstruction.
2. Branch-duct IPMN (approximately 57% of IPMNs): Pancreatic cysts (>5 mm) that communicate with the main pancreatic duct.
3. Mixed type IPMN (approximately 18% of IPMNs): Meets criteria for both main and branch duct.
Due to the asymptomatic nature of the disease, the overall incidence of IPMNs is difficult to define but is thought to account for approximately 3% to 5% of all pancreatic tumors. Most IPMNs are discovered as incidental lesions from the workup of an unrelated process by imaging or endoscopy. IPMNs are slightly more prevalent in males than in females, with a peak incidence of 60 to 70 years of age. Branch-duct IPMNs tend to occur in a slightly younger population and are less associated with malignancy compared with main-duct or mixed variants.
Because a majority of IPMNs are discovered incidentally, most are asymptomatic. When symptoms do occur, they tend to be nonspecific and include unexplained weight loss, anorexia, abdominal pain, and back pain. Jaundice can occur with mucin obstructing the ampulla or with an underlying invasive carcinoma. The obstruction of the pancreatic duct can also lead to pancreatitis. IPMNs may represent genomic instability of the entire pancreas. This concept, known as a “field defect,” has been described as a theoretical risk of developing a recurrent IPMN or pancreatic adenocarcinoma at a site remote from the original IPMN. The three different types of IPMNs, main duct, branch duct, and mixed duct, dictate different treatment algorithms.
MAIN DUCT IPMNs
Main-duct IPMNs should be resected in all patients unless the risks of existing comorbidities outweigh the benefits of resection. The goal of operative management of IPMNs is to remove all adenomatous or potentially malignant epithelium to minimize recurrence in the pancreas remnant. There are two theories on the pathophysiologic basis of IPMNs. The first groups IPMNs into a similar category as an adenocarcinoma, a localized process involving only a particular segment of the pancreas. The thought is that removal of the IPMN is the only treatment necessary. In contrast, some believe IPMNs to represent a field defect of the pancreas. All of the ductal epithelium remains at risk of malignant degeneration despite removal of the cyst. Ideally, a total pancreatectomy would eliminate all risk, but this is a radical procedure that is associated with metabolic derangements and exocrine insufficiency. Total pancreatectomy should be limited to the most fit patients, with a thorough preoperative assessment and proper risk stratification prior to undertaking this surgery.
There is less uncertainty with treatment of main-duct IPMNs. The high incidence of underlying malignancy associated with the IPMNs warrants surgical resection. IPMNs localized to the body and tail (approximately 33%) can undergo a distal pancreatectomy with splenectomy. At the time of surgery, a frozen section of the proximal margin should be interpreted by a pathologist to rule out high-grade dysplasia. A prospective study identified a concordance rate of 94% between frozen section and final pathologic examination. If the margin is positive (high-grade dysplasia, invasion) additional margins may be resected from the pancreas until no evidence of disease is present. However, most surgeons will proceed to a total pancreatectomy after two subsequent margins demonstrate malignant changes. This more extensive procedure should be discussed with the patient prior to surgery, and the patient should be properly consented regarding the risks of a total pancreatectomy.
IPMNs localized to the head or uncinate process of the pancreas should undergo a pancreaticoduodenectomy. A frozen section of the distal margin should be analyzed by pathology for evidence of disease. As mentioned before, after two additional margins reveal malignant changes, a total pancreatectomy is usually indicated (approximately 5%). The absence of abnormal changes in frozen sections does not equate to negative disease throughout the pancreas remnant. Rather, skip lesions involving the remainder of the pancreas can exist and thus patients ultimately still require imaging surveillance after successful resection. A prophylactic total pancreatectomy is rarely performed because the subsequent pancreatic endocrine (diabetes mellitus) and exocrine deficits (malnutrition) carry an increased morbidity.
BRANCH DUCT IPMNs
Localized branch-duct IPMN can be treated with a formal anatomic pancreatectomy, pancreaticoduodenectomy, or distal pancreatectomy, depending on the location of the lesion. However, guidelines were established that allow for nonoperative management with certain branch- type IPMN characteristics.
These include asymptomatic patients with a cyst size less than 3 cm and lack of mural nodules. The data to support this demonstrate a very low incidence of malignancy (approximately 2%) in this patient group. Which nearly matches the anticipated mortality of undergoing a formal anatomic resection. In approximately 20% to 30% of patients with branch- duct IPMNs, there is evidence of multifocality. The additional IPMNs can be visualized on high-resolution CT or MRI imaging. Ideally, patients with multifocal branch-duct IPMNs should undergo a total pancreatectomy. However, as previously mentioned, the increased morbidity and lifestyle alterations associated with a total pancreatectomy allows for a more conservative approach. This would include removing the most suspicious or dominant of the lesions in an anatomic resection and follow-up imaging surveillance of the remaining pancreas remnant. If subsequent imaging demonstrates malignant charac- teristics, a completion pancreatectomy is usually indicated.
RECURRENCE RATES
Recurrence rates with IPMNs are variable. An anatomic resection of a branch-duct IPMN with negative margins has been shown to be curative. The recurrence of a main- duct IPMN in the remnant gland is anywhere from 0% to 10% if the margins are negative and there is no evidence of invasion. Most case series cite a 5-year survival rate of at least 70% after resection of noninvasive IPMNs. In contrast, evidence of invasive disease, despite negative margins, decreases 5-year survival to 30% to 50%. The recurrence rate in either the pancreatic remnant or distant sites approaches 50% to 90% in these patients. Histopathologic subtype of the IPMN is correlated with survival. The aggressive tubular subtype has a 5-year survival ranging from 37% to 55% following surgical resection, whereas the colloid subtype has 5-year survival ranging from 61% to 87% post resection. Factors associated with decreased survival include tubular subtype, lymph node metastases, vascular invasion, and positive margins. IPMNs with evidence of invasion should be treated similar to pancreatic adenocarcinomas. Studies show that IPMNs tend to have better survival than pancreatic adenocarcinoma. This survival benefit may be secondary to the less aggressive tumor biology or the earlier diagnosis of IPMNs.
SURVEILLANCE
All patients who have a resected IPMN should undergo imaging surveillance. There is continual survival benefit with further resection if an IPMN does recur. International Consensus Guidelines published in 2017 offer recom- mendations for the frequency and modality of imaging surveillance after resection. Routine serum measurement of CEA and CA 19-9 has a limited role for detection of an IPMN recurrence. Of note, a new pancreatic lesion discovered on imaging after resection could represent a postoperative pseudocyst, a recurrence of the IPMN from inadequate resection, a new IPMN, or an unrelated new neoplastic process. IPMNs may also be associated with extrapancreatic neoplasms (stomach, colon, rectum, lung, breast) and pancreatic ductal adenocarcinoma. It is unclear if this represents a true genetic syndrome. However, patients with IPMNs should have a discussion about the implications of their disease with their physician and are encouraged to undergo colonoscopy to exclude a synchronous neoplastic process.
The incidence of PANCREATIC CYSTIC LESIONS will continue to increase as imaging technology improves. EUS, cytology, and molecular panels have made differentiating the type of PCN less problematic. The importance of an accurate preoperative diagnosis ensures that operative management is selectively offered to those with high-risk lesions. Management beyond surgery, including adjuvant therapy and surveillance, continue to be active areas of research.
Perioperative Medicine

Surgery and anesthesia profoundly alter the normal physiologic and metabolic states. Estimating the patient’s ability to respond to these stresses in the postoperative period is the task of the preoperative evaluation. Perioperative complications are often the result of failure, in the preoperative period, to identify underlying medical conditions, maximize the patient’s preoperative health, or accurately assess perioperative risk. Sophisticated laboratory studies and specialized testing are no substitute for a thoughtful and careful history and physical examination. Sophisticated technology has merit primarily in confirming clinical suspicion.
Classroom: Perioperative Medicine
Hepatocellular Carcinoma: Resection Versus Transplantation

Hepatocellular carcinoma is the second most common cause of cancer mortality worldwide and its incidence is rising in North America, with an estimated 35,000 cases in the U.S. in 2014. The best chance for cure is surgical resection in the form of either segmental removal or whole organ transplantation although recent survival data on radiofrequency ablation approximates surgical resection and could be placed under the new moniker of “thermal resection”. The debate between surgical resection and transplantation focuses on patients with “within Milan criteria” tumors, single tumors, and well compensated cirrhosis who can safely undergo either procedure. Although transplantation historically has had better survival outcomes, early diagnosis, reversal of liver disease, and innovations in patient selection and neo-adjuvant therapies have led to similar 5-year survival. Transplantation clearly has less risk of tumor recurrence but exposes recipients to long term immunosuppression and its side effects. Liver transplantation is also limited by the severe global limit on the supply of organ donors whereas resection is readily available. The current data does not favor one treatment over the other for patients with minimal or no portal hypertension and normal synthetic function. Instead, the decision to resect or transplant for HCC relies on multiple factors including tumor characteristics, biology, geography, co-morbidities, location, organ availability, social support and practice preference.
Resection Versus Transplantation
The debate between resection and transplantation revolves around patients who have well compensated cirrhosis with Milan criteria resectable tumors. Patients within these criteria represent a very small proportion of those who initially present with HCC. This is especially true in western countries where hepatitis C is the most common cause of liver failure and HCC is a result of the progressive and in most cases advanced cirrhosis.
Given the need for a large number of patients to show statistical significance, it would be difficult to perform a high-quality prospective randomized controlled trial comparing resection and transplantation. In fact the literature revealed that no randomized controlled trials addressing this issue exist. Instead, outcomes of surgical treatment for HCC stem from retrospective analyses that have inherent detection, selection and attrition biases.
Given the numerous articles available on this subject, several meta-analyses have been published to delineate the role of transplantation and resection for treatment of HCC. However, there is reason to be wary of these meta-analyses because they pool data from heterogeneous populations with variable selection criteria and treatment protocols. One such meta-analysis by Dhir et al. focused their choice of articles to strict criteria which excluded studies with non-cirrhotic patients, fibrolamellar HCC and hepato-cholangiocarcinomas but included those with HCC within Milan criteria and computation of 5-year survival; between 1990 and 2011 they identified ten articles that fit within these criteria, of which six were ITT analyses, six included only well-compensated cirrhotics (Child-Pugh Class A without liver dysfunction) and three were ITT analyses of well-compensated cirrhotics.
Analysis of the six ITT studies that included all cirrhotics (n = 1118) (Child-Pugh Class A through C) showed no significant difference in survival at 5 years (OR = 0.600, 95 % CI 0.291– 1.237 l; p=0.166) but ITT analysis of only well-compensated cirrhotics (Child- Pugh Class A) revealed that patients undergoing transplant had a significantly higher 5-year survival as compared to those with resection (OR=0.521, 95 % CI 0.298–0.911; p=0.022).
A more recent ITT retrospective analysis from Spain assessed long-term survival and tumor recurrence following resection or transplant for tumors <5 cm in 217 cirrhotics (Child-Pugh Class A, B and C) over the span of 16 years. Recurrence at 5 years was significantly higher in the resection group (71.6 % vs. 16 % p<0.001) but survival at 4 years was similar (60 % vs. 62 %) which is likely explained by the evolving role of adjuvant therapies to treat post-resection recurrence.
Conclusions
- Patients with anatomically resectable single tumors and no cirrhosis or Child-Pugh Class A cirrhosis with normal bilirubin, HVPG (<10 mmHg), albumin and INR can be offered resection (evidence quality moderate; strong recommendation).
- Patients with Milan criteria tumors in the setting of Child- Pugh Class A with low platelets and either low albumin or high bilirubin or Child-Pugh Class B and C cirrhosis, especially those with more than one tumor, should be offered liver transplantation over resection (evidence quality moderate; strong recommendation).
- Those with Milan criteria tumors and Child-Pugh Class A cirrhosis without liver dysfunction should be considered for transplantation over resection (evidence quality low; weak recommendation).
- No recommendation can be made in regard to transplanting tumors beyond Milan criteria (evidence quality low) except to follow regional review board criteria.
- Pre-transplant therapies such as embolic or thermal ablation are safe and by expert opinion considered to be effective in decreasing transplant waitlist dropout and bridging patients to transplant (evidence quality low, weak recommendation). These interventions should be considered for those waiting longer than 6 months (evi- dence quality low, moderate recommendation).
- Living donor liver transplantation is a safe and effective option for treatment of HCC that are within and exceed Milan criteria (evidence quality moderate, weak recommendation).
How to Choose a Mesh in Hernia Repair

Since the introduction of polypropylene (PP) mesh for hernia repair, surgeons continue to discuss the use of mesh in a variety of settings for one of the most common operations performed by general surgeons—hernia repair. This discussion has involved raw materials, cost, and outcomes and for many years referred to only a few products, as manufacturing was limited. Nowadays, with multiple permanent, absorbable, biologic, and hybrid products on the market, the choice of mesh for a hernia repair can be daunting. Increasing clinical complexity further emphasizes the need for individualizing care, but more frequently, hospital supply chain personnel institute product procurement procedures for cost control, limiting mesh choice for surgeons. This can force surgeons into a “one-size-fits-all” practice regarding mesh choice, which may not be ideal for some patients. Conversely, current literature lacks definitive evidence supporting the use of one mesh over another, a fact that has not escaped the radar screen of the hospital supply chain and mesh industry, both of which attempt to limit vendor and mesh choice for financial gain. It is unlikely that this type of “proof” will ever come to fruition. This leaves us with choosing a mesh based on an algorithm that is centered on the patient and the patient’s unique clinical scenario.
Article: Mesh in Hernia Repair
The Surgical Personality

Surgical stereotypes are remnants of the days of pre-anaesthesia surgery and include impulsivity, narcissism, authoritativeness, decisiveness, and thinking hierarchically. Medical students hold these stereotypes of surgeons early in their medical training. As Pearl Katz says in the The Scalpel’s Edge: ‘Each generation perpetuates the culture and passes it on by recruiting surgical residents who appear to resemble them and training these residents to emulate their thinking and behaviour.’ The culture of surgery has evolved, and certain behaviours are rightly no longer seen as acceptable, Non-technical skills such as leadership and communication have become incorporated into surgical training. Wen Shen, Associate Professor of Clinical Surgery at University of California San Francisco, argues that this has gone too far: ‘Putting likeability before surgical outcomes is like judging a restaurant by the waiters and ignoring the food,’ I would argue that operative and communication skills are indivisible, An aggressive surgeon is a threat to patient safety if colleagues are frightened to speak up for fear of a colleague shouting or, worse, throwing instruments. Conversely, a flattened hierarchy promotes patient safety.
Read More
Article: The Surgical Personality
The “GOOD” Surgeon
Surgery is an extremely enjoyable, intellectually demanding and satisfying career, and many more people apply to become surgeons each year than there are available places.
Those who are successful have to be ready not just to learn a great deal, but have the right kind of personality for the job.
Is a surgical career right for you?
Read the link…
THE GOOD SURGEON

Modern Concepts of Pancreatic Surgery
 Operations on the gallbladder and bile ducts are among the surgical procedures most commonly performed by general surgeons. In most hospitals, cholecystectomy is the most frequently performed operation within the abdomen. Pancreatic surgery is less frequent , but because of the close relation between the biliary system and the pancreas, knowledge of pancreatic problems is equally essential to the surgeon. Acute and chronic pancreatitis and cancer of the pancreas are often encountered by surgeons, with apparently increasing frequency; their treatment remains difficult and perplexing. This review demonstrates the modern aspects of pancreatic surgery. Good study.
Operations on the gallbladder and bile ducts are among the surgical procedures most commonly performed by general surgeons. In most hospitals, cholecystectomy is the most frequently performed operation within the abdomen. Pancreatic surgery is less frequent , but because of the close relation between the biliary system and the pancreas, knowledge of pancreatic problems is equally essential to the surgeon. Acute and chronic pancreatitis and cancer of the pancreas are often encountered by surgeons, with apparently increasing frequency; their treatment remains difficult and perplexing. This review demonstrates the modern aspects of pancreatic surgery. Good study.
AULA: PRÍNCIPIOS MODERNOS DA CIRURGIA PANCREÁTICA
Palestras e Vídeoaulas

Vejam nos links a seguir algumas de nossas palestras disponíveis para download no Canal do SlideShare e Videoaulas presentes no You Tube.
Postoperative Delirium

Postoperative delirium is recognized as the most common surgical complication in older adults,occurring in 5% to 50% of older patients after an operation. With more than one-third of all inpatient operations in the United States being performed on patients 65 years or older, it is imperative that clinicians caring for surgical patients understand optimal delirium care. Delirium is a serious complication for older adults because an episode of delirium can initiate a cascade of deleterious clinical events, including other major postoperative complications, prolonged hospitalization, loss of functional independence, reduced cognitive function, and death. The annual cost of delirium in the United States is estimated to be $150 billion. Delirium is particularly compelling as a quality improvement target, because it is preventable in up to 40% of patients; therefore, it is an ideal candidate for preventive interventions targeted to improve the outcomes of older adults in the perioperative setting. Delirium diagnosis and treatment are essential components of optimal surgical care of older adults, yet the topic of delirium is under-represented in surgical teaching.
Postoperative Delirium in Older Adults
Surgical treatment of ACUTE PANCREATITIS
Acute pancreatitis is more of a range of diseases than it is a single pathologic entity. Its clinical manifestations range from mild, perhaps even subclinical, symptoms to a life-threatening or life-ending process. The classification of acute pancreatitis and its forms are discussed in fuller detail by Sarr and colleagues elsewhere in this issue. For the purposes of this discussion, the focus is on the operative interventions for acute pancreatitis and its attendant disorders. The most important thing to consider when contemplating operative management for acute pancreatitis is that we do not operate as much for the acute inflammatory process as for the complications that may arise from inflammation of the pancreas. In brieSurgical treatment of acute pancreatitisf, the complications are related to: necrosis of the parenchyma, infection of the pancreas or surrounding tissue, failure of pancreatic juice to safely find its way to the lumen of the alimentary tract, erosion into vascular or other structures, and a persistent systemic inflammatory state. The operations may be divided into three major categories: those designed to ameliorate the emergent problems associated with the ongoing inflammatory state, those designed to ameliorate chronic sequelae of an inflammatory event, and those designed to prevent a subsequent episode of acute pancreatitis. This article provides a review of the above.
SURGICAL TREATMENT OF ACUTE PANCREATITIS
O TEMPLO DO CIRURGIÃO.
Templo (do latim templum, “local sagrado”) é uma estrutura arquitetônica dedicada ao serviço religioso. O termo também pode ser usado em sentido figurado. Neste sentido, é o reflexo do mundo divino, a habitação de Deus sobre a terra, o lugar da Presença Real. É o resumo do macrocosmo e também a imagem do microcosmo: ‘o corpo é o templo do Espírito Santo’ (I, Coríntios, 6, 19).
Dos locais especiais, O corpo humano (morada da alma), a Cavidade Peritoneal e o Bloco Cirúrgico, se bem analisados, são muito semelhantes e merecem atitudes e comportamentos respeitáveis. O Templo, em todos os credos, induz à meditação, absoluto silêncio tentando ouvir o Ser Supremo. A cavidade peritoneal | abdominal , espaço imaculado da homeostase, quando injuriada, reage gritando em dor, implorando uma precoce e efetiva ação terapêutica.
O Bloco Cirúrgico, abrigo momentâneo do indivíduo solitário, que mudo e quase morto de medo, recorre à prece implorando a troca do acidente, da complicação, da recorrência, da seqüela, da mutilação, da iatrogenia e do risco de óbito pela agressiva e controlada intervenção que lhe restaure a saúde, patrimônio magno de todo ser vivo.
O Bloco Cirúrgico clama por respeito ao paciente cirúrgico, antes mesmo de ser tomado por local banal, misturando condutas vulgares, atitudes menores, desvio de comportamento e propósitos secundários. Trabalhar no Bloco Cirúrgico significa buscar a perfeição técnica, revivendo os ensinamentos de William Stewart Halsted , precursor da arte de operar, dissecando para facilitar, pinçando e ligando um vaso sangüíneo, removendo tecido macerado, evitando corpos estranhos e reduzindo espaço vazio, numa síntese feita com a ansiedade e vontade da primeira e a necessidade e experiência da última.
Mas, se a cirurgia e o cirurgião vêm sofrendo grande evolução, técnica a primeira e científica o segundo, desde o início do século, a imagem que todo doente faz persiste numa simbiose entre mitos e verdades. A cirurgia significa enfrentar ambiente desconhecido chamado “sala de cirurgia” onde a fobia ganha espaço rumo ao infinito. O medo ainda prepondera em muitos.
A confiança neste momento além de um reconhecimento é um troféu que o cirurgião recebe dos pacientes e seus familiares. Tanto a CONFIANÇA quanto a SEGURANÇA têm que ser preservadas a qualquer custo. Não podem correr o risco de serem corroídas por palavras e atitudes de qualquer membro da equipe cirúrgica. Não foi tarefa fácil transformar, para a população, o ato cirúrgico numa atividade científica, indispensável, útil e por demais segura. Da conquista da cirurgia, como excelente arma terapêutica para a manutenção de um alto padrão de qualidade técnica, resta a responsabilidade dos cirurgiões, os herdeiros do suor e sangue, que se iniciou com o trabalho desenvolvido por Billroth, Lister, Halsted, Moyniham, Kocher e uma legião de figuras humanas dignas do maior respeito, admiração e gratidão universal.
No ato operatório os pacientes SÃO TODOS SEMELHANTES EM SUAS DIFERENÇAS, desde a afecção, ao prognóstico, ao caráter da cirurgia e especialmente sua relação com o ato operatório. Logo, o cirurgião tem por dever de ofício entrar no bloco cirúrgico com esperança e não deve sair com dúvida. Nosso trabalho é de equipe, cada um contribui com uma parcela, maior ou menor, para a concretização do todo, do ato cirúrgico por completo, com muita dedicação, profissionalismo e sabedoria. Toda tarefa, da limpeza do chão ao ato de operar, num crescendo, se faz em função de cada um e em benefício da maioria, o mais perfeito possível e de uma só vez, quase sempre sem oportunidade de repetição e previsão de término.
O trabalho do CIRURGIÃO é feito com carinho, muita dignidade, humildade e executado em função da alegria do resultado obtido aliado a dimensão ética do dever cumprido que transcende a sua existência. A vida do cirurgião se materializa no ato operatório e o bloco cirúrgico, palco do nosso trabalho não tolera e jamais permite atitudes menores, inferiores, ambas prejudiciais a todos os pacientes e a cada cirurgião. Como ambiente de trabalho de uma equipe diversificada, precisamos manter, a todo custo, o controle de qualidade, eficiência, eficácia e efetividade técnina associados aos mais altos valores ético, pois lidamos com o que há de mais precioso da criação divina na Terra: O SER HUMANO.
“Tem presença de Deus, como já a tens. Ontem estive com um doente, um doente a quem quero com todo o meu coração de Pai, e compreendo o grande trabalho sacerdotal que os médicos levam a cabo. Mas não se ponham orgulhosos, porque todas as almas são sacerdotais. Devem pôr em prática esse sacerdócio! Ao lavares as mãos, ao vestires a bata, ao calçares as luvas, pensa em Deus, e pensa nesse sacerdócio real de que fala São Pedro, e então não se te meterá a rotina: farás bem aos corpos e às almas” São Josemaria Escriva
Bariatric Complications

Over the past decade, following the publication of several long-term outcome studies that showed a significant improvement in cardiovascular risk and mortality after bariatric surgery, the number of bariatric procedures being carried out annually in the UK has grown exponentially. Surgery remains the only way to produce significant, sustainable weight loss and resolution of comorbidities. Nevertheless, relatively few surgeons have developed an interest in this field. Most bariatric surgery is now performed in centres staffed by surgeons with a bariatric interest, usually as part of a multidisciplinary team.
The commonest weight loss procedures performed around the world at present are the gastric band, the gastric bypass and the sleeve gastrectomy. In very obese patients, an alternative operation is the duodenal switch, while the new ileal transposition procedure represents one of the few purely metabolic operations designed specifically for the treatment of type II diabetes. Older operations such as vertical banded gastroplasty and jejuno-ileal bypass are now obsolete, although patients who have undergone such procedures in the distant past may still present to hospital with complications. The main endoscopic option at present is insertion of a gastric balloon, with newer procedures like the endoscopic duodenojejunal barrier and gastric plication on the horizon. Implantable neuroregulatory devices (gastric ‘pacemakers’) represent a new direction for surgical weight control by harnessing neural feedback signals to help control eating.
It should be within the capability of any abdominal surgeon to manage the general complications of bariatric surgery, which include pulmonary atelectasis/pneumonia, intra-abdominal bleeding, anastomotic or staple-line leak with or without abscess formation, deep vein thrombosis (DVT)/pulmonary embolus and superficial wound infections. Patients may be expected to present with malaise, pallor, features of sepsis or obvious wound problems. However, clinical features may be difficult to recognise owing to body habitus. Abdominal distension, tenderness and guarding may be impossible to determine clinically due to the patient’s obesity. Pallor is non-specific. Fever and leucocytosis may be absent. Wound collections may be very deep. These complications in a bariatric patient should be actively sought with appropriate investigations. In particular, it is vital for life-threatening complications such as bleeding, sepsis and bowel obstruction to be recognised promptly and treated appropriately. A persistent tachycardia may be the only sign heralding significant complications and should always be taken seriously. It is useful to classify complications as ‘early’, ‘medium’ and ‘late’ because, from the receiving clinician’s point of view, the differential diagnosis will differ accordingly.
Complications of bariatric surgery presenting to the GENERAL SURGEON
A “PROFISSÃO” CIRÚRGICA
 “A arte de curar vem do coração e da mente mais do que das mãos.” – Hipócrates
“A arte de curar vem do coração e da mente mais do que das mãos.” – Hipócrates
Na complexa tapeçaria da sociedade moderna, as profissões desempenham papéis fundamentais na organização dos serviços necessários ao bem-estar coletivo. Definida pelo American College of Surgeons, uma profissão é um campo onde a maestria de um corpo complexo de conhecimento e habilidades é essencial. É uma vocação em que o conhecimento científico ou a prática de uma arte, fundamentada nesse conhecimento, é empregada em benefício dos outros. O compromisso com a competência, a integridade e a moralidade forma a base de um contrato social entre a profissão e a sociedade, que concede à profissão um monopólio sobre o uso de seu conhecimento, considerável autonomia na prática e o privilégio da auto-regulação. Em troca, a profissão deve prestar contas a quem serve e à sociedade como um todo.
Os Elementos Essenciais da Profissão
No cerne de toda profissão estão quatro elementos fundamentais:
- Monopólio do Conhecimento Especializado: Profissionais detêm o direito exclusivo de utilizar conhecimentos e habilidades especializados, o que lhes confere uma posição única na sociedade.
- Autonomia e Auto-Regulação: Em troca deste monopólio, profissionais desfrutam de uma relativa autonomia na prática e são responsáveis pela sua própria regulação.
- Serviço Altruísta: A profissão deve servir tanto indivíduos quanto a sociedade de forma altruísta, colocando o bem-estar do paciente acima de outros interesses.
- Responsabilidade pela Manutenção e Expansão do Conhecimento: Profissionais são responsáveis por atualizar e expandir continuamente seu conhecimento e habilidades.
O Que é Profissionalismo?
Profissionalismo descreve as qualidades cognitivas, morais e colegiais de um profissional. É o conjunto de razões pelas quais um pai se orgulha de dizer que seu filho é um médico e cirurgião. Profissionalismo é mais do que apenas conhecimento técnico; é uma combinação de ética, respeito e dedicação ao ofício e ao paciente.
Por Que Precisamos de um Código de Conduta Profissional?
A confiança é o alicerce da prática cirúrgica. O Código de Conduta Profissional esclarece a relação entre a profissão cirúrgica e a sociedade que serve, frequentemente referido como contrato social. Para os pacientes, o código cristaliza o compromisso da comunidade cirúrgica em relação aos indivíduos e suas comunidades. A confiança é construída, tijolo por tijolo.
O Código de Conduta Profissional
O Código de Conduta Profissional aplica os princípios gerais do profissionalismo à prática cirúrgica e serve como a fundação sobre a qual os privilégios profissionais e a confiança dos pacientes e do público são conquistados. Durante o cuidado pré-operatório, intraoperatório e pós-operatório, os cirurgiões têm a responsabilidade de:
- Advogar Eficazmente pelos interesses dos pacientes.
- Divulgar Opções Terapêuticas incluindo seus riscos e benefícios.
- Divulgar e Resolver Conflitos de Interesse que possam influenciar as decisões de cuidado.
- Ser Sensível e Respeitoso com os pacientes, compreendendo sua vulnerabilidade durante o período perioperatório.
- Divulgar Completamente Eventos Adversos e Erros Médicos.
- Reconhecer Necessidades Psicológicas, Sociais, Culturais e Espirituais dos pacientes.
- Incorporar Cuidados Especiais para Pacientes Terminais.
- Reconhecer e Apoiar as Necessidades das Famílias dos Pacientes.
- Respeitar o Conhecimento, Dignidade e Perspectiva de outros profissionais de saúde.
A Necessidade do Código de Profissionalismo para Cirurgiões
Procedimentos cirúrgicos são experiências extremas que impactam os pacientes fisiológica, psicológica e socialmente. Quando os pacientes se submetem a uma experiência cirúrgica, devem confiar que o cirurgião colocará seu bem-estar acima de todas as outras considerações. O código escrito ajuda a reforçar esses valores, garantindo que a confiança e o compromisso sejam mantidos.
Princípios Fundamentais do Código de Conduta Profissional
- Primazia do Bem-Estar do Paciente: Os interesses do paciente sempre devem vir em primeiro lugar. O altruísmo é central para esse conceito, e é o altruísmo do cirurgião que fomenta a confiança na relação médico-paciente.
- Autonomia do Paciente: Pacientes devem entender e tomar suas próprias decisões informadas sobre o tratamento. Os médicos devem ser honestos para que os pacientes façam escolhas educadas, garantindo que essas decisões estejam alinhadas com práticas éticas.
- Justiça Social: Como médicos, devemos advogar pelos pacientes individuais enquanto promovemos a saúde do sistema de saúde como um todo. Precisamos equilibrar as necessidades dos pacientes (autonomia) sem desviar recursos escassos que beneficiariam a sociedade (justiça social).
“Não há maior coisa a ser conquistada do que a confiança dos pacientes e da sociedade, pois ela é a base sobre a qual construímos nossas práticas e nossa profissão.” – William Osler
Metabolismo Perioperatório
A melhor forma de se conhecer as necessidades energéticas é através de sua medida por calorimetria indireta, cada vez mais disponível em nosso ambiente hospitalar. Quando não se dispõe de calorimetria indireta, é possível estimar o gasto energético por meio de fórmulas estimativas que levam em conta, entre outros fatores, o peso e altura corpóreos, idade e sexo. Das diferentes fórmulas disponíveis, a equação de Harris-Benedict tem sido muito usada. Para homens a formula é 66,5 + (13.8 x peso [kg])+(5,0 x altura [cm]) – (6,8 x idade [anos]). Para mulheres a formula é diferente: 655 + (9,6 x peso [kg]) + (1,7 x altura [cm]) – (4,7 x idade [anos]). A regra de bolso (30-35 kcal/kg/dia) é mais prática e também é muito utilizada. No período pré-operatório a oferta de proteína deve ser em torno de 1,0-1,5 g/Kg/dia e, após trauma ou intervenção cirúrgica aumenta, podendo chegar até 2,0 g/Kg/dia. Em pacientes com SIRS moderado, a oferta calórica deve ser menor (25-30 kcal/kg/dia). Pacientes em estresse importante (SIRS grave, sepse) devem receber 20-25 kal/kg/dia e 1,5 a 2,0 g de proteínas/kg/dia. Deve-se evitar em pacientes gravemente desnutridos aporte rápido de calorias e proteínas (síndrome da realimentação). Nesses pacientes a oferta deve ser cautelosa com controle diário de fósforo, magnésio e potássio.
Aula: Suporte Nutricional Perioperatório
Tratamento Cirúrgico da Hemorragia digestiva alta por varizes esofágicas | Hipertensão Porta
O sistema portal é uma rede venosa de baixa pressão, com níveis fisiológicos <5 mmHg. Desta forma, o termo hipertensão portal (HP) designa uma síndrome clínica caracterizada pelo aumento mantido na pressão venosa em níveis acima dos fisiológicos. Ela é considerada clinicamente significante quando acima de 10 mmHg; neste nível existe o risco de surgimento de varizes esofagogástricas (VEG). Por sua vez, valores acima de 12 mmHg cursam com risco de rompimento dessas varizes, sua principal complicação.
ARTIGO DE REVISÃO – HIPERTENSÃO PORTAL
O aumento do fluxo como fator preponderante inicial da HP é raro e representado por fístulas arterioportais congênitas, traumáticas ou neoplásicas. O aumento da resistência é a condição fisiopatológica inicial mais comum e pode ser classificada de acordo com o local de obstrução ao fluxo em: pré-hepática, intra-hepática e pós-hepática. A HP intra-hepática responde pela grande maioria dos casos e pode ser subdividida de acordo com o local de acometimento estrutural no parênquima hepático em: pré-sinusoidal (ex: esquistossomose hepatoesplênica – EHE), sinusoidal (ex: cirrose hepática) e pós-sinusoidal (ex: doença venoclusiva). Em nosso meio, a maioria dos casos é decorrente da EHE e das hepatopatias crônicas complicadas com cirrose.
O tratamento da HP depende da causa subjacente, da condição clínica e do momento em que é realizado. Pacientes com função hepática comprometida têm abordagem diversa daqueles com ela preservada, como os portadores de EHE. Além disso, o tratamento pode ser emergencial (durante episódio agudo de hemorragia) ou eletivo, como profilaxia pré-primária, primária ou secundária. Por essa diversidade de situações clínicas, não existe modalidade única de tratamento.
O objetivo da aula abaixo foi avaliar os avanços e as estratégias atuais empregadas no tratamento emergencial e eletivo da hemorragia digestiva varicosa em pacientes cirróticos e esquistossomóticos.
AULA: TRATAMENTO CIRÚRGICO DA HIPERTENSÃO PORTAL
FERIDA PÓS-OPERATÓRIA

A avaliação e os cuidados de feridas pós-operatórias deve ser do domínio de todos os profissionais que atuam na clínica cirúrgica. O conhecimento a cerca dos processos relacionados a cicatrização tecidual é importante tanto nos cuidados como na prevenção de complicações, tais como: infecções e deiscência. Como tal, todos os profissionais médicos, sendo eles cirurgiões ou de outras especialidades, que participam do manejo clínico dos pacientes no período perioperatório devem apreciar a fisiologia da cicatrização de feridas e os princípios de tratamento de feridas pós-operatório. O objetivo deste artigo é atualizar os profissionais médicos de outras especialidades sobre os aspectos importantes do tratamento de feridas pós-operatório através de uma revisão da fisiologia da cicatrização de feridas, os métodos de limpeza e curativo, bem como um guia sobre complicações de feridas pós-operatórias mais prevalentes e como devem ser manejados nesta situação.
Causas de conversão da VIDEOCOLECISTECTOMIA

Atualmente, a colecistectomia laparoscópica é a abordagem preferida para o tratamento da litíase biliar, representando cerca de 90% dos procedimentos realizados, uma marca alcançada nos Estados Unidos em 1992. A popularidade dessa técnica se deve a suas vantagens evidentes: menos dor no pós-operatório, recuperação mais rápida, redução dos dias de trabalho perdidos e menor tempo de hospitalização. Apesar de ser considerada o padrão-ouro na cirurgia biliar, a colecistectomia laparoscópica não está isenta de desafios. Entre 2% e 15% dos casos podem exigir a conversão para cirurgia convencional. Os motivos mais comuns para essa conversão incluem dificuldades na identificação da anatomia, suspeita de lesão da árvore biliar e controle de sangramentos. Identificar os fatores que contribuem para uma maior taxa de conversão é essencial para a equipe cirúrgica. Isso não apenas permite uma avaliação mais precisa da complexidade do procedimento, mas também ajuda na preparação do paciente para possíveis riscos e na mobilização de cirurgiões mais experientes quando necessário. Em um cenário onde a precisão e a segurança são cruciais, a compreensão dos desafios e a preparação adequada podem fazer toda a diferença no resultado da cirurgia.
Relacionados ao Paciente: 1. Obesidade (IMC > 35), 2. Sexo Masculino, 3. Idade > 65 anos, 4. Diabetes Mellitus e 5. ASA > 2.
Relacionadas a Doença: 1. Colecistite Aguda, 2. Líquido Pericolecístico, 3. Pós – CPRE, 4. Síndrome de Mirizzi e 5. Edema da parede da vesícula > 5 mm.
Relacionadas a Cirurgia: 1. Hemorragia, 2. Aderências firmes, 3. Anatomia obscura, 4. Fístulas internas e 5. Cirurgia abdominal prévia.
POST-HEPATECTOMY ADVERSE EVENTS
Hepatic resection had an impressive growth over time. It has been widely performed for the treatment of various liver diseases, such as malignant tumors, benign tumors, calculi in the intrahepatic ducts, hydatid disease, and abscesses. Management of hepatic resection is challenging. Despite technical advances and high experience of liver resection of specialized centers, it is still burdened by relatively high rates of postoperative morbidity and mortality. Especially, complex resections are being increasingly performed in high risk and older patient population. Operation on the liver is especially challenging because of its unique anatomic architecture and because of its vital functions. Common post-hepatectomy complications include venous catheter-related infection, pleural effusion, incisional infection, pulmonary atelectasis or infection, ascites, subphrenic infection, urinary tract infection, intraperitoneal hemorrhage, gastrointestinal tract bleeding, biliary tract hemorrhage, coagulation disorders, bile leakage, and liver failure. These problems are closely related to surgical manipulations, anesthesia, preoperative evaluation and preparation, and postoperative observation and management. The safety profile of hepatectomy probably can be improved if the surgeons and medical staff involved have comprehensive knowledge of the expected complications and expertise in their management.
Classroom: Hepatic Resections
The era of hepatic surgery began with a left lateral hepatic lobectomy performed successfully by Langenbuch in Germany in 1887. Since then, hepatectomy has been widely performed for the treatment of various liver diseases, such as malignant tumors, benign tumors, calculi in the intrahepatic ducts, hydatid disease, and abscesses. Operation on the liver is especially challenging because of its unique anatomic architecture and because of its vital functions. Despite technical advances and high experience of liver resection of specialized centers, it is still burdened by relatively high rates of postoperative morbidity (4.09%-47.7%) and mortality (0.24%-9.7%). This review article focuses on the major postoperative issues after hepatic resection and presents the current management.
REVIEW_ARTICLE_HEPATECTOMY_COMPLICATIONS
PANCREATIC PSEUDOCYST
Classroom: Principles of Pancreatic Surgery
The pancreatic pseudocyst is a collection of pancreatic secretions contained within a fibrous sac comprised of chronic inflammatory cells and fibroblasts in and adjacent to the pancreas contained by surrounding structures. Why a fibrous sac filled with pancreatic fluid is the source of so much interest, speculation, and emotion amongst surgeons and gastroenterologists is indeed hard to understand. Do we debate so vigorously about bilomas, urinomas, or other abdominal collections of visceral secretions? Perhaps it is because the pancreatic pseudocyst represents a sleeping tiger, which though frequently harmless, still can rise up unexpectedly and attack with its enzymatic claws into adjacent visceral and vascular structures and cause lifethreatening complications. Another part of the debate and puzzlement about pancreatic pseudocysts is related to confusion about pancreatic pseudocyst definition and nomenclature. The Atlanta classification, developed in 1992, was a pioneering effort in describing and defining morphologic entities in acute pancreatitis. Since then, a working group has been revising this system to incorporate more modern experience into the terminology. In the latest version of this system, pancreatitis is divided into acute interstitial edematous pancreatitis (IEP) and necrotizing pancreatitis (NP), based on the presence of pancreatic tissue necrosis. The fluid collections associated with these two “types” of pancreatitis are also differentiated. Early (<4 weeks into the disease course) peripancreatic fluid collections in IEP are referred to as acute peripancreatic fluid collections (APFC), whereas in NP, they are referred to as postnecrotic peripancreatic fluid collections (PNPFC). Late (>4 weeks) fluid collections in IEP are called pancreatic pseudocysts, and in NP, they are called walled-off pancreatic necrosis (WOPN).
Review of POSTGASTRECTOMY SYNDROMES

The first postgastrectomy syndrome was noted not long after the first gastrectomy was performed: Billroth reported a case of epigastric pain associated with bilious vomiting as a sequel of gastric surgery in 1885. Several classic treatises exist on the subject; we cannot improve on them and merely provide a few references for the interested reader. Surgical procedures on the stomach, performed for reasons such as peptic ulcer disease, cancer, obesity, or gastroesophageal reflux disease, can result in various post-gastrectomy syndromes. These syndromes include chronic symptoms that range from mild discomfort to life-altering conditions. This guide covers the most common syndromes and their characteristics.
GASTRECTOMY VIDEO SURGERY
Dumping Syndrome
Dumping Syndrome is characterized by gastrointestinal and vasomotor symptoms that occur after food intake due to rapid gastric emptying. This syndrome can occur after surgeries that alter the regulation of gastric emptying or gastric compliance, such as gastrectomy, proximal vagotomy, sleeve gastrectomy, fundoplication, pyloroplasty, and gastrojejunostomy (GJ). Depending on the speed of emptying and the osmolarity of gastric contents, symptoms can vary.
- Early Dumping: Occurs within 30 minutes after food intake and is characterized by palpitations, tachycardia, fatigue, a need to lie down after meals, flushing or pallor, sweating, dizziness, hypotension, headache, and possibly syncope. Abdominal symptoms include early satiety, epigastric fullness, abdominal pain, bloating, hypermotility, and splenic blood pooling.
- Late Dumping: Appears 1 to 3 hours after eating, due to reactive hypoglycemia caused by an initially high glucose load leading to an inappropriately high insulin response. Symptoms include sweating, faintness, difficulty concentrating, and altered levels of consciousness.
Diagnosis is confirmed through an oral glucose tolerance test or a gastric emptying scintigraphy study.
Post-Vagotomy Diarrhea
Post-vagotomy diarrhea is a common complication after vagotomy, characterized by frequent episodes of watery diarrhea. It can be attributed to changes in intestinal motility and bile secretion.
Gastric Stasis
Gastric stasis or delayed gastric emptying can occur due to disruption of normal gastric motility. Symptoms include nausea, vomiting, and a feeling of fullness. Diagnosis is confirmed through gastric emptying studies.
Bile Reflux Gastritis
Bile reflux gastritis is caused by the reflux of bile into the stomach, resulting in epigastric pain and bilious vomiting. Diagnosis can be confirmed through upper endoscopy and gastric pH monitoring.
Afferent and Efferent Loop Syndromes
Afferent loop syndrome occurs after Billroth II reconstruction and is characterized by abdominal pain, bilious vomiting, and distention. Efferent loop syndrome occurs when there is an obstruction of the efferent loop, leading to similar symptoms.
Roux Syndrome
Roux syndrome is a complication of Roux-en-Y procedures, characterized by postprandial abdominal pain and vomiting. Diagnosis is made through a contrast gastrointestinal transit study.
Therapeutic Approach
Management of post-gastrectomy syndromes includes dietary modifications, such as eating small frequent meals, separating liquids and solids, increasing protein and fat intake, and reducing simple sugars. In some cases, additional pharmacological or surgical interventions may be necessary. Understanding these syndromes and their therapeutic approaches is crucial to providing effective care and improving the quality of life for post-gastrectomy patients.
This article focuses on the small proportion of patients with severe, debilitating symptoms; these symptoms can challenge the acumen of the surgeon who is providing the patient’s long-term follow-up and care.
POSTGASTRECTOMY_SYNDROMES_REVIEW_ARTICLE
Complications of HEMORROIDH SURGERY
Symptomatic hemorrhoids require a number of therapeutic interventions each of which has its own complications. Office-based therapy such as rubber band ligation carries the risk of pain and bleeding, which are self-limited, but also carries the risk of rare complications such as sepsis, which may be life threatening. Operative treatment of hemorrhoids includes conventional hemorrhoidectomy, stapled hemorrhoidectomy, and the use of energy devices. Complications of pain and bleeding are common but self-limited. Late complications such as stenosis and fecal incontinence are rare. Recurrent disease is related to the initial grade and therapeutic approach. Treatment of recurrent hemorrhoids should be individualized based on previous treatments and the grade of disease. Anesthetic complications, especially urinary retention, are common and related to the anesthetic technique. Practitioners should council their patients as to the risks of the various approaches to treating symptomatic hemorrhoids.
Intra Abdominal Infections
With intra-abdominal infection being one of the most common reasons for surgical consultation, understanding the evaluation and management of these processes becomes paramount in the day- to-day practice of the surgeon. The very broad nature of who is affected coupled with the interplay of patient comorbidities and their medications make dealing with intra-abdominal infections a challenge. As with most complex problems in medicine, it is often useful to break them down into simpler and smaller parts. One useful way to categorize intra-abdominal infections is to divide them into those originating from previous abdominal trauma or operations and those presenting in a “virgin” abdomen.
The latter group most commonly includes those patients presenting with specific organ-based infectious processes such as appendicitis, cholecystitis, or diverticulitis. These individual diseases are covered extensively in other chapters and are discussed only superficially in this chapter. The former are those patients who have sustained intra-abdominal trauma or have undergone previous abdominal interventions and are not recovering in the usual expected course. It is this group that taxes diagnostic and clinical skills and may require the most complex medical decision making.
Several factors should come into play once suspicion for an intra-abdominal infection is entertained. These include resuscitation, antibiotic usage, and source control itself. Patients who present with either a suspected or diagnosed intra-abdominal infection should have some form of volume resuscitation. Even without hypotension, there are several reasons why these patients might be volume depleted. These include nausea and vomiting, fluid sequestration within the abdominal cavity or lumen of the bowel, and poor oral intake. As the process progresses, the patient may develop tachypnea, which results in an evaporative fluid loss. By this time, one can often elicit orthostatic hypotension in most patients.
Fluid resuscitation should begin with the administration of isotonic crystalloid and in general be guided by evidence of end organ perfusion (adequate mental status, urine output, correction of acidosis). There is no utility-using colloid such as albumin or hetastarch in these circumstances, and some data suggest a worse outcome. Should the patient present with hypotension or evidence of poor perfusion, a more aggressive approach to volume resuscitation should be employed. Our recommendation is to follow the current surviving sepsis guidelines, which include fluid challenges, monitoring/assessment of filling pressures, and the potential use of pressors and steroids.
KIDNEY INJURY on perioperative period
ACUTE KIDNEY FAILURE_REVIEW ARTICLE
Alterations in renal function are common after surgical emergencies, trauma, and major operations. In these settings, successful recovery of renal function is dependent on prompt diagnosis and protective management strategies. Acute kidney injury (AKI) is characterized by an acute decrease in glomerular filtration rate (GFR). The true incidence of AKI and acute renal failure (ARF) has been difficult to define, given the broad and various definitions used to quantify and study altered renal function. Relatively recent introduction of consensus definitions, such as RIFLE (risk, failure, loss, and end-stage renal failure) criteria and AKIN (Acute Kidney Injury Network) staging, have provided standard definitions to facilitate more uniform outcome reporting. With use of these definitions, recent studies suggest that AKI occurs in up to two thirds of patients in the intensive care unit (ICU). Moreover, increasing severity of AKI is associated with increasing mortality. AKI is also associated with increased morbidity, such as increased hospital length of stay and cost of care, and has been linked to other in-hospital complications, such as increased difficulty in weaning from mechanical ventilation. Preoperative risk factors for development of AKI include older age, emergent surgery, hepatic disease, obesity, high-risk surgery, vascular disease, and chronic obstructive pulmonary disease (COPD). Prompt recognition of AKI facilitates effective treatment. Although the incidence rate of AKI appears to be rising, overall outcomes from AKI are gradually improving.
The reported mortality rate of AKI is 30% to 60%. If RRT is necessary, reported mortality rates are over 50%. The reason for such high mortality is that AKI now usually occurs as part of a spectrum of multiple organ failure, most often associated with severe sepsis or septic shock. The mortality in this setting is often determined by the underlying septic syndrome, rather than by complications of individual organ failure. Of surviving patients of AKI, a significant number have development of chronic renal insufficiency, which necessitates chronic dialysis. The precise rate of development of chronic renal failure varies greatly in the literature, depending on the patient populations. A recent review of AKI estimates that overall, the risk of necessary chronic dialysis is approximately 12%.
Laparoscopic Surgery for Morbid Obesity
The morbid obesity epidemic continues to spread throughout industrialized nations. It is a condition with a heterogeneous etiology, including genetic, psychosocial, and environmental factors. Prevention methods have currently been unable to halt the further spread of this disease. Obesity has been linked to increased healthcare costs, common physiologic derangements, reduced quality of life, and increased overall mortality. More than one third of adults and almost 17% of children in the United States are obese.
Medical therapy that can cause sustained significant weight loss may be years away. Bariatric surgery, when combined with a multidisciplinary team, continues to be the only proven method to achieve sustained weight loss in most patients. Bariatric procedures modify gastrointestinal anatomy and, in some cases, enteric hormone release to reduce caloric intake, reduce absorption, and alter metabolism to achieve weight loss. Currently, the three most common bariatric operations in the United States are Roux-en-Y gastric bypass, adjustable gastric band, and the vertical sleeve gastrectomy.
GOSSIPIBOMA
O termo “gossipiboma” refere-se a uma matriz de matéria têxtil envolvida por reacção de corpo estranho. O termo é derivado do latim “Gossypium”, algodão, e o Swahili “boma”, que significa “esconderijo”. Também conhecida como textiloma, originada de “textilis” (tecer em latim) e “oma” (doença, tumor ou inchaço em grego). O primeiro caso foi descrito por Wilson em 1884. Gossipibomas foram relatados após operações em muitos processos, e em diferentes órgãos e localização. Mas, o local mais comum é o abdominal. Gaze e compressas são os materiais mais comumente retidos após laparotomia. A incidência de gossipibomas é variável e subnotificada, principalmente devido às implicações legais de sua detecção, mas também porque muitos pacientes permanecem assintomáticos. A apresentação clínica é também variável. O tratamento recomendado é a excisão que pretende evitar as complicações que conduzem a taxa de mortalidade entre11-35%.
Epidemiologia
Ele ocorre entre 1/1000 a 1/1500 nas operações intra-abdominais. A apresentação clínica é variável e depende da localização do corpo estranho e sobre o tipo de reação inflamatória apresentada pela hospedeiro. Podem existir formas agudas e crônicas. A forma aguda tende a apresentar-se com fístulas e abcessos cutâneos, enquanto que a crônica como massa encapsulada (granuloma de corpo estranho) e sintomas inespecíficos. Gossipibomas ocorrem mais comumente após operação abdominal e pélvica. Eles são mais frequentes em pacientes obesos e quando a operação é realizada em emergência. A incidência é maior em nove vezes após operação de emergência, e de quatro em procedimentos não planejados no decorrer de uma intervenção, mudando o que se pretendia realizar. Outros fatores predisponentes incluem operações em campo de batalha, complicações intra-operatórias, tais como perda intensa de sangue, a incapacidade de realizar contagem de materiais cirúrgicos no final do processo, tempo de operação prolongado e as mudanças no pessoal médico e de enfermagem durante o operação.
Evolução clínica
O tempo entre a operação e aparecimento de manifestações clínicas de Gossipiboma é variável, em particular se o material permanecer estéril. Ele depende da localização do material retido e do tipo de reação orgânica, e foi estimado em entre 10 dias a vários anos. Em patologia, duas reações de corpo estranho pode occorer. A primeira resposta é a produção asséptica de fibrina, o que leva à formação de aderências, material de encapsulamento e à formação de granulomas de corpo estranho. Nesta apresentação, o paciente pode permanecer assintomático por meses ou anos. A segunda resposta é exsudativa, com formação de abcessos, fístulas aos órgãos internos como o estômago, intestino, bexiga, cólon ou vagina, ou também fístula externa para a parede abdominal. Os sintomas dependem do órgão afetado principalmente e podem resultar da compressão, obstrução, síndrome de má absorção, ou crescimento bacteriano. Eles incluem dor abdominal, tumor palpável, náuseas, vômitos, sangramento retal, diarréia, disúria, piúria, hematúria e urgência urinária. Os sintomas sistêmicos como febre, anorexia, anemia e perda de peso também podem occurer. No entanto, a resposta inflamatória e aderências podem formar uma cápsula com o bloqueio omental e órgãos adjacentes, podendo o paciente permanecer assintomático. A falta de sintomas pode dificultar ou retardar o diagnóstico, que muitas vezes é realizado incidentalmente.
Possibilidades diagnósticas
O diagnóstico pode ser difícil. Suspeita clínica e o uso de estudos de imagem são importantes, pois é a regra a inexistência ou inespecificidade de sintomas em vários anos após a operação. No pré-operatório pode ser levantada suspeita por meio de estudos radiológicos ou endoscópicos. Muitos casos só são descobertos no intra-operatório. Tomografia computadorizada é o exame complementar de escolha para o diagnóstico e avaliação dessas complicações. Ele fornece informações detalhadas sobre a lesão na maioria dos casos. A aparência pode ser lesão cística espongiforme, cápsula hiperdensa em camadas concêntricas, ou calcificações murais. A presença de gás é indicativa de perfuração do intestino ou à formação de abcessos. Os principais diagnósticos diferenciais são: aderências pós-operatórias, fecalomas, contusões, hematomas, intussuscepção, volvo, tumores e abscessos intracavitários.
Tratamento e Prognóstico
O tratamento de escolha é a remoção cirúrgica que pode ser realizada por laparoscopia ou laparotomia, e visa prevenir complicações. O prognóstico da gossipiboma é variável com taxas de mortalidade de 11 para 35%. Quando a remoção ocorre no período pós-operatório imediato, a morbidade e mortalidade são baixas; no entanto, se o material foi mantido por um longo tempo a remoção pode exigir operação extensa e ter elevado índice de complicações.
Implicações médico-legais
Há muitas implicações médico-legais com gossypiboma. Revisão de negligência médica impetradas entre 1988 e 1994 revelou 40 casos de gossipiboma, que representaram 48% de todos os corpos estranhos. Não foi possível determinar se o material esquecimento representou falta de qualidade do cirurgião ou quadro de enfermagem.
Procedimentos preventivos
A abordagem mais importante é a prevenção. As medidas preventivas necessárias incluem o uso de material têxtil com marcadores radiopacos e contagem minuciosa de materiais cirúrgicos. São recomendadas quatro contagens: na montagem do material, antes da operação, no início do fechamento da cavidade e durante a síntese da pele. Dhillon e Park reforçam a importância da exploração dos quatro quadrantes abdominais no final da operação em todos os casos, mesmo após a contagem das compressas. No caso de contagem incorreta, a menos que o paciente seja considerado instável, a síntese da cavidade não deve ser realizada até que todas elas estejam localizados.
CONCLUSÃO
Gossipiboma é um problema médico-legal sério e sua incidência está aparentemente aumentando. Por isso, os meios e métodos nos procedimentos cirúrgicos durante o ato operatório e no contexto geral da sala de operações precisam ser revistos para tomarem-se medidas preventivas. Formação continuada de profissionais da área médica e estrita adesão à técnica operatória são primordiais para a prevenção de gossipiboma.
Abdominal Hernia Surgical EMERGENCIES
A hernia is a weakness or disruption of the fibromuscular tissues through which an internal organ (or part of the organ) protrudes or slides through. Collectively, inguinal and femoral hernias are often lumped together into groin hernias. Surgery remains the only effective treatment, but the optimal timing and method of repair remain controversial. Although strangulation rates of 3% at 3 months have been reported by some investigators, the largest prospective randomized trial of (watchful waiting) men with minimally symptomatic inguinal hernias showed that watchful waiting is safe. Frequency of strangulation was only 2.4% in patients followed up for as long as 11.5 years. Long-term follow-up shows that more than two-thirds of men using a strategy of watchful waiting cross over to surgical repair, with pain being the most common reasons. This risk of crossover is higher in patients older than 65 years. Once an inguinal hernia becomes symptomatic, surgical repair is clearly indicated. Femoral hernias are more likely to present with strangulation and require emergency surgery and are thus repaired even when asymptomatic. Because this article focuses on incarcerated hernias, nonoperative options are not discussed.